Bacteriophages to Control Multi-Drug Resistant Enterococcus faecalis Infection of Dental Root Canals
- PMID: 33802385
- PMCID: PMC7998577
- DOI: 10.3390/microorganisms9030517
Bacteriophages to Control Multi-Drug Resistant Enterococcus faecalis Infection of Dental Root Canals
Abstract
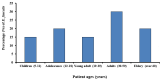
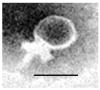
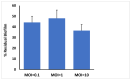
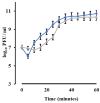
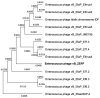
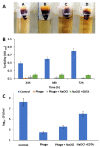
Phage therapy is an alternative treatment to antibiotics that can overcome multi-drug resistant bacteria. In this study, we aimed to isolate and characterize lytic bacteriophages targeted against Enterococcus faecalis isolated from root canal infections obtained from clinics at the Faculty of Dentistry, Ismalia, Egypt. Bacteriophage, vB_ZEFP, was isolated from concentrated wastewater collected from hospital sewage. Morphological and genomic analysis revealed that the phage belongs to the Podoviridae family with a linear double-stranded DNA genome, consisting of 18,454, with a G + C content of 32.8%. Host range analysis revealed the phage could infect 10 of 13 E. faecalis isolates exhibiting a range of antibiotic resistances recovered from infected root canals with efficiency of plating values above 0.5. One-step growth curves of this phage showed that it has a burst size of 110 PFU per infected cell, with a latent period of 10 min. The lytic activity of this phage against E. faecalis biofilms showed that the phage was able to control the growth of E. faecalis in vitro. Phage vB_ZEFP could also prevent ex-vivo E. faecalis root canal infection. These results suggest that phage vB_ZEFP has potential for application in phage therapy and specifically in the prevention of infection after root canal treatment.
Keywords: Enterococcus faecalis; bacteriophage; multi-drug resistant; phage therapy; root canal infection.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

