Tumor Growth Rate Estimates Are Independently Predictive of Therapy Response and Survival in Recurrent High-Grade Serous Ovarian Cancer Patients
- PMID: 33802395
- PMCID: PMC7959281
- DOI: 10.3390/cancers13051076
Tumor Growth Rate Estimates Are Independently Predictive of Therapy Response and Survival in Recurrent High-Grade Serous Ovarian Cancer Patients
Abstract
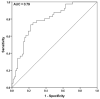
This study aimed to assess the predictive value of tumor growth rate estimates based on serial cancer antigen-125 (CA-125) levels on therapy response and survival of patients with recurrent high-grade serous ovarian cancer (HGSOC). In total, 301 consecutive patients with advanced HGSOC (exploratory cohort: n = 155, treated at the Medical University of Vienna; external validation cohort: n = 146, from the Ovarian Cancer Therapy-Innovative Models Prolong Survival (OCTIPS) consortium) were enrolled. Tumor growth estimates were obtained using a validated two-phase equation model involving serial CA-125 levels, and their predictive value with respect to treatment response to the next chemotherapy and the prognostic value with respect to disease-specific survival and overall survival were assessed. Tumor growth estimates were an independent predictor for response to second-line chemotherapy and an independent prognostic factor for second-line chemotherapy use in both univariate and multivariable analyses, outperforming both the predictive (second line: p = 0.003, HR 5.19 [1.73-15.58] vs. p = 0.453, HR 1.95 [0.34-11.17]) and prognostic values (second line: p = 0.042, HR 1.53 [1.02-2.31] vs. p = 0.331, HR 1.39 [0.71-2.27]) of a therapy-free interval (TFI) < 6 months. Tumor growth estimates were a predictive factor for response to third- and fourth-line chemotherapy and a prognostic factor for third- and fourth-line chemotherapy use in the univariate analysis. The CA-125-derived tumor growth rate estimate may be a quantifiable and easily assessable surrogate to TFI in treatment decision making for patients with recurrent HGSOC.
Keywords: growth rate; ovarian cancer; platinum-resistant; recurrence; therapy response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Colombo N., Sessa C., Bois A.d., Ledermann J., Mcluggage W.G., McNeish I., Morice P., Pignata S., Ray-Coquard I., Vergote I., et al. ESMO–ESGO consensus conference recom-mendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019;30:672–705. doi: 10.1093/annonc/mdz062. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

