Dehydropeptide Supramolecular Hydrogels and Nanostructures as Potential Peptidomimetic Biomedical Materials
- PMID: 33802425
- PMCID: PMC7959283
- DOI: 10.3390/ijms22052528
Dehydropeptide Supramolecular Hydrogels and Nanostructures as Potential Peptidomimetic Biomedical Materials
Abstract
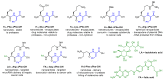
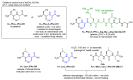
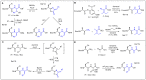
Supramolecular peptide hydrogels are gaining increased attention, owing to their potential in a variety of biomedical applications. Their physical properties are similar to those of the extracellular matrix (ECM), which is key to their applications in the cell culture of specialized cells, tissue engineering, skin regeneration, and wound healing. The structure of these hydrogels usually consists of a di- or tripeptide capped on the N-terminus with a hydrophobic aromatic group, such as Fmoc or naphthalene. Although these peptide conjugates can offer advantages over other types of gelators such as cross-linked polymers, they usually possess the limitation of being particularly sensitive to proteolysis by endogenous proteases. One of the strategies reported that can overcome this barrier is to use a peptidomimetic strategy, in which natural amino acids are switched for non-proteinogenic analogues, such as D-amino acids, β-amino acids, or dehydroamino acids. Such peptides usually possess much greater resistance to enzymatic hydrolysis. Peptides containing dehydroamino acids, i.e., dehydropeptides, are particularly interesting, as the presence of the double bond also introduces a conformational restraint to the peptide backbone, resulting in (often predictable) changes to the secondary structure of the peptide. This review focuses on peptide hydrogels and related nanostructures, where α,β-didehydro-α-amino acids have been successfully incorporated into the structure of peptide hydrogelators, and the resulting properties are discussed in terms of their potential biomedical applications. Where appropriate, their properties are compared with those of the corresponding peptide hydrogelator composed of canonical amino acids. In a wider context, we consider the presence of dehydroamino acids in natural compounds and medicinally important compounds as well as their limitations, and we consider some of the synthetic strategies for obtaining dehydropeptides. Finally, we consider the future direction for this research area.
Keywords: cancer; dehydrodipeptide; drug delivery; hydrogel; peptidomimetic; smart materials; supramolecular; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Johnson E.K., Adams D.J., Cameron P.J. Peptide based low molecular weight gelators. J. Mater. Chem. 2011;21:2024–2027. doi: 10.1039/C0JM03099F. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

