Cell Death and Inflammation: The Role of Mitochondria in Health and Disease
- PMID: 33802550
- PMCID: PMC7998762
- DOI: 10.3390/cells10030537
Cell Death and Inflammation: The Role of Mitochondria in Health and Disease
Abstract
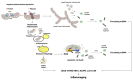
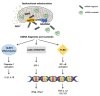
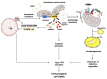
Mitochondria serve as a hub for a multitude of vital cellular processes. To ensure an efficient deployment of mitochondrial tasks, organelle homeostasis needs to be preserved. Mitochondrial quality control (MQC) mechanisms (i.e., mitochondrial dynamics, biogenesis, proteostasis, and autophagy) are in place to safeguard organelle integrity and functionality. Defective MQC has been reported in several conditions characterized by chronic low-grade inflammation. In this context, the displacement of mitochondrial components, including mitochondrial DNA (mtDNA), into the extracellular compartment is a possible factor eliciting an innate immune response. The presence of bacterial-like CpG islands in mtDNA makes this molecule recognized as a damaged-associated molecular pattern by the innate immune system. Following cell death-triggering stressors, mtDNA can be released from the cell and ignite inflammation via several pathways. Crosstalk between autophagy and apoptosis has emerged as a pivotal factor for the regulation of mtDNA release, cell's fate, and inflammation. The repression of mtDNA-mediated interferon production, a powerful driver of immunological cell death, is also regulated by autophagy-apoptosis crosstalk. Interferon production during mtDNA-mediated inflammation may be exploited for the elimination of dying cells and their conversion into elements driving anti-tumor immunity.
Keywords: apoptosis; damage-associated molecular patterns (DAMPs); immunogenic cell death; innate immunity; mitochondrial dynamics; mitochondrial dysfunction; mitochondrial quality control (MQC); mitophagy; oxidative stress; reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Picca A., Guerra F., Calvani R., Bucci C., Monaco M.R., Lo Bentivoglio A.R., Coelho-Júnior H.J., Landi F., Bernabei R., Marzetti E. Mitochondrial dysfunction and aging: Insights from the analysis of extracellular vesicles. Int. J. Mol. Sci. 2019;20:805. doi: 10.3390/ijms20040805. - DOI - PMC - PubMed
-
- Picca A., Lezza A.M.S., Leeuwenburgh C., Pesce V., Calvani R., Bossola M., Manes-Gravina E., Landi F., Bernabei R., Marzetti E. Circulating mitochondrial DNA at the crossroads of mitochondrial dysfunction and inflammation during aging and muscle wasting disorders. Rejuvenation Res. 2018;21:350–359. doi: 10.1089/rej.2017.1989. - DOI - PMC - PubMed
-
- Caielli S., Athale S., Domic B., Murat E., Chandra M., Banchereau R., Baisch J., Phelps K., Clayton S., Gong M., et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 2016;213:697–713. doi: 10.1084/jem.20151876. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

