Disruption of Endoplasmic Reticulum and ROS Production in Human Ovarian Cancer by Campesterol
- PMID: 33802602
- PMCID: PMC8001332
- DOI: 10.3390/antiox10030379
Disruption of Endoplasmic Reticulum and ROS Production in Human Ovarian Cancer by Campesterol
Abstract
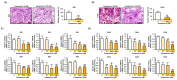
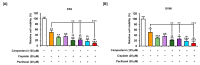
Phytosterols, which are present in a variety of foods, exhibit various physiological functions and do not have any side effects. Here, we attempted to identify functional role of campesterol in regulation of oxidative stress by leading to cell death of ovarian cancer. We investigated the effects of campesterol on cancer cell aggregation using a three-dimensional (3D) culture of human ovarian cancer cells. The effects of campesterol on apoptosis, protein expression, proliferation, the cell cycle, and the migration of these cells were determined to unravel the underlying mechanism. We also investigated whether campesterol regulates mitochondrial function, the generation of reactive oxygen species (ROS), and calcium concentrations. Our results show that campesterol activates cell death signals and cell death in human ovarian cancer cells. Excessive calcium levels and ROS production were induced by campesterol in the two selected ovarian cancer cell lines. Moreover, campesterol suppressed cell proliferation, cell cycle progression, and cell aggregation in ovarian cancer cells. Campesterol also enhanced the anticancer effects of conventional anticancer agents. The present study shows that campesterol can be used as a novel anticancer drug for human ovarian cancer.
Keywords: ROS; campesterol; cell death; mitochondria dysfunction; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

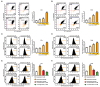
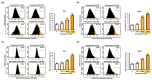
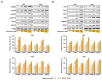
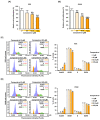

References
-
- Genser B., Silbernagel G., De Backer G., Bruckert E., Carmena R., Chapman M.J., Deanfield J., Descamps O.S., Rietzschel E.R., Dias K.C., et al. Plant sterols and cardiovascular disease: A systematic review and meta-analysis†. Eur. Hear. J. 2012;33:444–451. doi: 10.1093/eurheartj/ehr441. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

