New Insights into the Metabolism of Methyltestosterone and Metandienone: Detection of Novel A-Ring Reduced Metabolites
- PMID: 33802606
- PMCID: PMC7961831
- DOI: 10.3390/molecules26051354
New Insights into the Metabolism of Methyltestosterone and Metandienone: Detection of Novel A-Ring Reduced Metabolites
Abstract
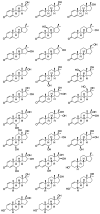
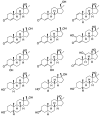
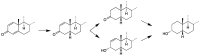
Metandienone and methyltestosterone are orally active anabolic-androgenic steroids with a 17α-methyl structure that are prohibited in sports but are frequently detected in anti-doping analysis. Following the previously reported detection of long-term metabolites with a 17ξ-hydroxymethyl-17ξ-methyl-18-nor-5ξ-androst-13-en-3ξ-ol structure in the chlorinated metandienone analog dehydrochloromethyltestosterone ("oral turinabol"), in this study we investigated the formation of similar metabolites of metandienone and 17α-methyltestosterone with a rearranged D-ring and a fully reduced A-ring. Using a semi-targeted approach including the synthesis of reference compounds, two diastereomeric substances, viz. 17α-hydroxymethyl-17β-methyl-18-nor-5β-androst-13-en-3α-ol and its 5α-analog, were identified following an administration of methyltestosterone. In post-administration urines of metandienone, only the 5β-metabolite was detected. Additionally, 3α,5β-tetrahydro-epi-methyltestosterone was identified in the urines of both administrations besides the classical metabolites included in the screening procedures. Besides their applicability for anti-doping analysis, the results provide new insights into the metabolism of 17α-methyl steroids with respect to the order of reductions in the A-ring, the participation of different enzymes, and alterations to the D-ring.
Keywords: 17-hydroxymethyl-17-methyl-18-nor; 17α-methyl steroids; D-ring alteration; doping control; gas chromatography-mass spectrometry; long-term metabolites; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



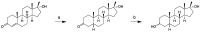



Similar articles
-
Reduced and rearranged metabolite structures after metandienone administration: New promising metabolites for potential long-term detection.J Steroid Biochem Mol Biol. 2025 Oct;253:106801. doi: 10.1016/j.jsbmb.2025.106801. Epub 2025 Jun 3. J Steroid Biochem Mol Biol. 2025. PMID: 40473058
-
New long-standing metabolites of 17α-methyltestosterone are detected in HepG2 cell in vitro metabolic model and in human urine.Drug Test Anal. 2024 Jun;16(6):604-615. doi: 10.1002/dta.3589. Epub 2023 Oct 30. Drug Test Anal. 2024. PMID: 37903531
-
Detection and mass spectrometric characterization of novel long-term dehydrochloromethyltestosterone metabolites in human urine.J Steroid Biochem Mol Biol. 2012 Feb;128(3-5):121-7. doi: 10.1016/j.jsbmb.2011.11.004. Epub 2011 Nov 27. J Steroid Biochem Mol Biol. 2012. PMID: 22142641
-
Factors influencing the steroid profile in doping control analysis.J Mass Spectrom. 2008 Jul;43(7):877-91. doi: 10.1002/jms.1457. J Mass Spectrom. 2008. PMID: 18570179 Review.
-
Ultra high performance liquid chromatography tandem mass spectrometry determination and profiling of prohibited steroids in human biological matrices. A review.J Chromatogr B Analyt Technol Biomed Life Sci. 2013 May 15;927:22-36. doi: 10.1016/j.jchromb.2012.12.003. Epub 2012 Dec 20. J Chromatogr B Analyt Technol Biomed Life Sci. 2013. PMID: 23317577 Review.
Cited by
-
Breaking the Bottleneck in Anticancer Drug Development: Efficient Utilization of Synthetic Biology.Molecules. 2022 Nov 2;27(21):7480. doi: 10.3390/molecules27217480. Molecules. 2022. PMID: 36364307 Free PMC article. Review.
-
Medaka embryos as a model for metabolism of anabolic steroids.Arch Toxicol. 2022 Jul;96(7):1963-1974. doi: 10.1007/s00204-022-03284-4. Epub 2022 Mar 30. Arch Toxicol. 2022. PMID: 35352155 Free PMC article.
References
-
- Whitelaw M.J., Foster T.N., Graham W.H. Methandrostenolone (Dianabol): A controlled study of its anabolic and androgenic effect in children. J. Pediatrics. 1966;68:294–295. doi: 10.1016/S0022-3476(66)80161-0. - DOI
-
- World Anti-Doping Agency The 2021 Prohibited List. [(accessed on 16 February 2021)]; Available online: https://www.wada-ama.org/sites/default/files/resources/files/2021list_en....
-
- World Anti-Doping Agency . Anti-Doping Testing Figures—Laboratory Report. World Anti-Doping Agency; Montreal, QC, Canada: 2019.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

