Therapeutic Role of Tocilizumab in SARS-CoV-2-Induced Cytokine Storm: Rationale and Current Evidence
- PMID: 33802761
- PMCID: PMC8002419
- DOI: 10.3390/ijms22063059
Therapeutic Role of Tocilizumab in SARS-CoV-2-Induced Cytokine Storm: Rationale and Current Evidence
Abstract
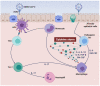
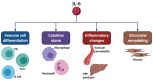
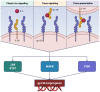
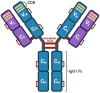
Among patients suffering from coronavirus disease 2019 (COVID-19) syndrome, one of the worst possible scenarios is represented by the critical lung damage caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)-induced cytokine storm, responsible for a potentially very dangerous hyperinflammatory condition. Within such a context, interleukin-6 (IL-6) plays a key pathogenic role, thus being a suitable therapeutic target. Indeed, the IL-6-receptor antagonist tocilizumab, already approved for treatment of refractory rheumatoid arthritis, is often used to treat patients with severe COVID-19 symptoms and lung involvement. Therefore, the aim of this review article is to focus on the rationale of tocilizumab utilization in the SARS-CoV-2-triggered cytokine storm, as well as to discuss current evidence and future perspectives, especially with regard to ongoing trials referring to the evaluation of tocilizumab's therapeutic effects in patients with life-threatening SARS-CoV-2 infection.
Keywords: ARDS; IL-6; SARS-CoV-2; cytokine storm; tocilizumab.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

