Crocetin Mitigates Irradiation Injury in an In Vitro Model of the Pubertal Testis: Focus on Biological Effects and Molecular Mechanisms
- PMID: 33802807
- PMCID: PMC8002482
- DOI: 10.3390/molecules26061676
Crocetin Mitigates Irradiation Injury in an In Vitro Model of the Pubertal Testis: Focus on Biological Effects and Molecular Mechanisms
Abstract
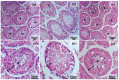
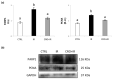
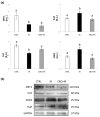
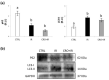
Infertility is a potential side effect of radiotherapy and significantly affects the quality of life for adolescent cancer survivors. Very few studies have addressed in pubertal models the mechanistic events that could be targeted to provide protection from gonadotoxicity and data on potential radioprotective treatments in this peculiar period of life are elusive. In this study, we utilized an in vitro model of the mouse pubertal testis to investigate the efficacy of crocetin to counteract ionizing radiation (IR)-induced injury and potential underlying mechanisms. Present experiments provide evidence that exposure of testis fragments from pubertal mice to 2 Gy X-rays induced extensive structural and cellular damage associated with overexpression of PARP1, PCNA, SOD2 and HuR and decreased levels of SIRT1 and catalase. A twenty-four hr exposure to 50 μM crocetin pre- and post-IR significantly reduced testis injury and modulated the response to DNA damage and oxidative stress. Nevertheless, crocetin treatment did not counteract the radiation-induced changes in the expression of SIRT1, p62 and LC3II. These results increase the knowledge of mechanisms underlying radiation damage in pubertal testis and establish the use of crocetin as a fertoprotective agent against IR deleterious effects in pubertal period.
Keywords: HuR; SIRT1; X-rays; autophagy; crocetin; fertility preservation; oxidative stress; pubertal testis; radiotherapy; saffron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

