Ex-Situ Evaluation of Commercial Polymer Membranes for Vanadium Redox Flow Batteries (VRFBs)
- PMID: 33802914
- PMCID: PMC8002826
- DOI: 10.3390/polym13060926
Ex-Situ Evaluation of Commercial Polymer Membranes for Vanadium Redox Flow Batteries (VRFBs)
Abstract
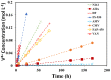
Polymer membranes play a vital role in vanadium redox flow batteries (VRFBs), acting as a separator between the two compartments, an electronic insulator for maintaining electrical neutrality of the cell, and an ionic conductor for allowing the transport of ionic charge carriers. It is a major influencer of VRFB performance, but also identified as one of the major factors limiting the large-scale implementation of VRFB technology in energy storage applications due to its cost and durability. In this work, five (5) high-priority characteristics of membranes related to VRFB performance were selected as major considerable factors for membrane screening before in-situ testing. Eight (8) state-of-the-art of commercially available ion exchange membranes (IEMs) were specifically selected, evaluated and compared by a set of ex-situ assessment approaches to determine the possibility of the membranes applied for VRFB. The results recommend perfluorosulfonic acid (PFSA) membranes and hydrocarbon anion exchange membranes (AEMs) as the candidates for further in-situ testing, while one hydrocarbon cation exchange membrane (CEM) is not recommended for VRFB application due to its relatively high VO2+ ion crossover and low mechanical stability during/after the chemical stability test. This work could provide VRFB researchers and industry a valuable reference for selecting the polymer membrane materials before VRFB in-situ testing.
Keywords: chemical stability; ex-situ evaluation; membrane; proton conductivity; vanadium ion crossover; vanadium redox flow batteries (VRFBs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ye R.J., Henkensmeier D., Yoon S.J., Huang Z.F., Kim D.K., Chang Z.J., Kim S., Chen R.Y. Redox Flow Batteries for Energy Storage: A Technology Review. J. Electrochem. Energy Convers. Storage. 2018;15 doi: 10.1115/1.4037248. - DOI
-
- Ulaganathan M., Aravindan V., Yan Q.Y., Madhavi S., Skyllas-Kazacos M., Lim T.M. Recent Advancements in All-Vanadium Redox Flow Batteries. Adv. Mater. Interfaces. 2016;3 doi: 10.1002/admi.201500309. - DOI
-
- Minke C., Kunz U., Turek T. Techno-economic assessment of novel vanadium redox flow batteries with large-area cells. J. Power Sources. 2017;361:105–114. doi: 10.1016/j.jpowsour.2017.06.066. - DOI
-
- Yuan X.Z., Song C.J., Platt A., Zhao N.N., Wang H.J., Li H., Fatih K., Jang D. A review of all-vanadium redox flow battery durability: Degradation mechanisms and mitigation strategies. Int. J. Energy Res. 2019;43:6599–6638. doi: 10.1002/er.4607. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

