Self-Reported Real-World Safety and Reactogenicity of COVID-19 Vaccines: A Vaccine Recipient Survey
- PMID: 33803014
- PMCID: PMC8002738
- DOI: 10.3390/life11030249
Self-Reported Real-World Safety and Reactogenicity of COVID-19 Vaccines: A Vaccine Recipient Survey
Abstract
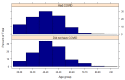
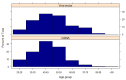
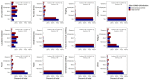
An online survey was conducted to compare the safety, tolerability and reactogenicity of available COVID-19 vaccines in different recipient groups. This survey was launched in February 2021 and ran for 11 days. Recipients of a first COVID-19 vaccine dose ≥7 days prior to survey completion were eligible. The incidence and severity of vaccination side effects were assessed. The survey was completed by 2002 respondents of whom 26.6% had a prior COVID-19 infection. A prior COVID-19 infection was associated with an increased risk of any side effect (risk ratio 1.08, 95% confidence intervals (1.05-1.11)), fever (2.24 (1.86-2.70)), breathlessness (2.05 (1.28-3.29)), flu-like illness (1.78 (1.51-2.10)), fatigue (1.34 (1.20-1.49)) and local reactions (1.10 (1.06-1.15)). It was also associated with an increased risk of severe side effects leading to hospital care (1.56 (1.14-2.12)). While mRNA vaccines were associated with a higher incidence of any side effect (1.06 (1.01-1.11)) compared with viral vector-based vaccines, these were generally milder (p < 0.001), mostly local reactions. Importantly, mRNA vaccine recipients reported a considerably lower incidence of systemic reactions (RR < 0.6) including anaphylaxis, swelling, flu-like illness, breathlessness and fatigue and of side effects requiring hospital care (0.42 (0.31-0.58)). Our study confirms the findings of recent randomised controlled trials (RCTs) demonstrating that COVID-19 vaccines are generally safe with limited severe side effects. For the first time, our study links prior COVID-19 illness with an increased incidence of vaccination side effects and demonstrates that mRNA vaccines cause milder, less frequent systemic side effects but more local reactions.
Keywords: COVID-19; COVID-19 vaccine; Coronavirus Disease 2019; adverse events; reactogenicity; safety; tolerability.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form. None of the authors has any conflict of interest in relation to this work. A.G.M. reports grants from Boehringer Ingelheim outside the submitted work. R.B. reports contract research on behalf of Public Health England for GlaxoSmithKline, Pfizer and Sanofi Pasteur outside the submitted work. N.D.B. reports personal fees from TEVA Pharma, GSK, AstraZeneca, Boehringer Ingelheim and Chiesi Pharma outside the submitted work.
Figures

References
-
- Arnold D.T., Hamilton F.W., Milne A., Morley A.J., Viner J., Attwood M., Noel A., Gunning S., Hatrick J., Hamilton S., et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax. 2020;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. - DOI - PMC - PubMed
-
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

