Pyrrole-Aminopyrimidine Ensembles: Cycloaddition of Guanidine to Acylethynylpyrroles
- PMID: 33803018
- PMCID: PMC8002744
- DOI: 10.3390/molecules26061692
Pyrrole-Aminopyrimidine Ensembles: Cycloaddition of Guanidine to Acylethynylpyrroles
Abstract
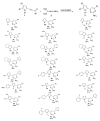
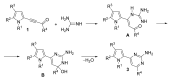
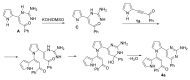
An efficient method for the synthesis of pharmaceutically prospective pyrrole-aminopyrimidine ensembles (in up to 91% yield) by the cyclocondensation of easily available acylethynylpyrroles with guanidine nitrate has been developed. The reaction proceeds under heating (110-115 °C, 4 h) in the KOH/DMSO system. In the case of 2-benzoylethynylpyrrole, the unexpected addition of the formed pyrrole-aminopyrimidine as N- (NH moiety of the pyrrole ring) and C- (CH of aminopyrimidine) nucleophiles to the triple bond is observed.
Keywords: acylethynylpyrroles; aminopyrimidine; cyclocondensation; guanidine.
Conflict of interest statement
Authors reports no conflict interests.
Figures





References
-
- Jones R.A., Bean G.P. The Chemistry of Pyrroles. Academic Press; London, UK: 1977. p. 525.
-
- Bhardwaj V., Gumber D., Abbot V., Dhiman S., Sharma P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015;5:15233–15266. doi: 10.1039/C4RA15710A. - DOI
-
- Koroleva E.V., Gusak K.N., Ignatovich Z.V. Synthesis and applications of 2-aminopyrimidine derivatives as key intermediates in chemical synthesis of biomolecules. Russ. Chem. Rev. 2010;79:655–681. doi: 10.1070/RC2010v079n08ABEH004116. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

