In-Vitro and In-Silico Assessment of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous Film-Forming Foam (AFFF) Binding to Human Serum Albumin
- PMID: 33803062
- PMCID: PMC8002870
- DOI: 10.3390/toxics9030063
In-Vitro and In-Silico Assessment of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous Film-Forming Foam (AFFF) Binding to Human Serum Albumin
Erratum in
-
Correction: Li et al. In-Vitro and In-Silico Assessment of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous Film-Forming Foam (AFFF) Binding to Human Serum Albumin. Toxics 2021, 9, 63.Toxics. 2022 May 31;10(6):297. doi: 10.3390/toxics10060297. Toxics. 2022. PMID: 35736949 Free PMC article.
Abstract
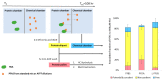
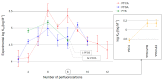
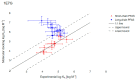
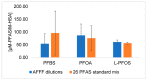
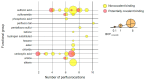
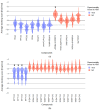
Drinking water contaminated by fluorosurfactant-based aqueous film-forming foams (AFFF) is a source of human exposure to poly- and perfluoroalkyl substances (PFAS). However, assessment of bioaccumulation potentials of diverse PFAS in commercial products such as AFFF have been insufficient and challenging, especially due to a lack of analytical standards. Here we explore the value of suspect screening, equilibrium dialysis, and molecular-docking simulations to identify potentially bioaccumulative PFAS. We exposed human serum albumin (HSA) protein to dilutions of a legacy AFFF produced by 3M in 1999 using equilibrium dialysis and screened in-vitro protein-binding affinities using high-resolution mass spectrometry (HRMS). Through suspect screening, we identified 32 PFAS and 18 hydrocarbon surfactants in the AFFF that bound to HSA. Quantification of noncovalent association constants for 26 PFAS standards confirmed that many PFAS, including the short-chain perfluoropropane sulfonic acid (log Ka= 4.1 ± 0.2 M-1), exhibit strong binding affinities with HSA. At least five PFAS in AFFF (including three PFAS with less than five perfluorocarbons) remained bound to the precipitated HSA pellet after extensive solvent washing-an indication of high PFAS binding potential. Three PFAS (PFBS, PFOS, and PFOA) were confirmed in the protein pellet with analytical standards and quantified after acid digestion-this sample fraction accounted for 5 to 20% of each compound mass in the sample. We calculated pseudo-bioconcentration factors (BCFpseudo) for PFAS that suspect screening flagged as noncovalently bound or potentially covalently bound. Most PFAS exhibiting high BCFpseudo, especially those with seven perfluorocarbons, contained a carboxylic acid or a sulfonic acid. Finally, we used molecular docking to simulate HSA binding affinities for 62 ligands (26 PFAS targets, 18 PFAS qualified in AFFF, and 18 hydrocarbon surfactants qualified in AFFF). We found that molecular docking can effectively separate HSA-binding and -nonbinding compounds in AFFF. In-vitro and in-silico approaches described in this study provide replicable, high-throughput workflows for assessing bioaccumulation potentials of diverse PFAS in commercial products.
Keywords: PFAS; bioconcentration; docking; equilibrium dialysis; suspect screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Fate and Transformation of 15 Classes of Per- and Polyfluoroalkyl Substances in Aqueous Film-Forming Foam (AFFF)-Amended Soil Microcosms.Environ Sci Technol. 2024 Dec 24;58(51):22777-22789. doi: 10.1021/acs.est.4c08665. Epub 2024 Dec 10. Environ Sci Technol. 2024. PMID: 39654523
-
Developing potency factors for thyroid hormone disruption by PFASs using TTR-TRβ CALUX® bioassay and assessment of PFASs mixtures in technical products.Environ Int. 2021 Dec;157:106791. doi: 10.1016/j.envint.2021.106791. Epub 2021 Aug 4. Environ Int. 2021. PMID: 34364217
-
Chemical Characterization of a Legacy Aqueous Film-Forming Foam Sample and Developmental Toxicity in Zebrafish (Danio rerio).Environ Health Perspect. 2020 Sep;128(9):97006. doi: 10.1289/EHP6470. Epub 2020 Sep 23. Environ Health Perspect. 2020. PMID: 32966100 Free PMC article.
-
A review of the occurrence and microbial transformation of per- and polyfluoroalkyl substances (PFAS) in aqueous film-forming foam (AFFF)-impacted environments.Sci Total Environ. 2024 Jun 1;927:171883. doi: 10.1016/j.scitotenv.2024.171883. Epub 2024 Mar 24. Sci Total Environ. 2024. PMID: 38531439 Review.
-
Concentration profiles of per- and polyfluoroalkyl substances in major sources to the environment.J Environ Manage. 2022 Jan 1;301:113879. doi: 10.1016/j.jenvman.2021.113879. Epub 2021 Oct 4. J Environ Manage. 2022. PMID: 34619593 Review.
Cited by
-
Advances in waste-derived functional materials for PFAS remediation.Biodegradation. 2025 Jan 20;36(1):13. doi: 10.1007/s10532-025-10109-5. Biodegradation. 2025. PMID: 39832063 Review.
-
Broad PFAS Binding with Fatty Acid Binding Protein 4 Is Enabled by Variable Binding Modes.JACS Au. 2025 Jun 2;5(6):2469-2474. doi: 10.1021/jacsau.5c00504. eCollection 2025 Jun 23. JACS Au. 2025. PMID: 40575325 Free PMC article.
-
An in vitro and machine learning framework for quantifying serum albumin binding of per- and polyfluoroalkyl substances.Toxicol Sci. 2025 Jan 1;203(1):67-78. doi: 10.1093/toxsci/kfae124. Toxicol Sci. 2025. PMID: 39298512 Free PMC article.
-
Broad PFAS binding with fatty acid binding protein 4 is enabled by variable binding modes.bioRxiv [Preprint]. 2025 Jan 14:2025.01.10.632451. doi: 10.1101/2025.01.10.632451. bioRxiv. 2025. Update in: JACS Au. 2025 Jun 02;5(6):2469-2474. doi: 10.1021/jacsau.5c00504. PMID: 40196552 Free PMC article. Updated. Preprint.
-
Role of Mineral-Organic Interactions in PFAS Retention by AFFF-Impacted Soil.Environ Sci Technol. 2023 Apr 4;57(13):5231-5242. doi: 10.1021/acs.est.2c08806. Epub 2023 Mar 22. Environ Sci Technol. 2023. PMID: 36947878 Free PMC article.
References
-
- Barzen-Hanson K.A., Roberts S.C., Choyke S., Oetjen K., McAlees A., Riddell N., McCrindle R., Ferguson P.L., Higgins C.P., Field J.A. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017;51:2047–2057. doi: 10.1021/acs.est.6b05843. - DOI - PubMed
-
- Gyllenhammar I., Berger U., Sundström M., McCleaf P., Eurén K., Eriksson S., Ahlgren S., Lignell S., Aune M., Kotova N., et al. Influence of Contaminated Drinking Water on Perfluoroalkyl Acid Levels in Human Serum—A Case Study from Uppsala, Sweden. Environ. Res. 2015;140:673–683. doi: 10.1016/j.envres.2015.05.019. - DOI - PubMed
-
- Graber J.M., Alexander C., Laumbach R.J., Black K., Strickland P.O., Georgopoulos P.G., Marshall E.G., Shendell D.G., Alderson D., Mi Z., et al. Per- and Polyfluoroalkyl Substances (PFAS) Blood Levels after Contamination of a Community Water Supply and Comparison with 2013-14 NHANES. J. Expo. Sci. Environ. Epidemiol. 2019;29:172–182. doi: 10.1038/s41370-018-0096-z. - DOI - PMC - PubMed
-
- Daniels R.D., Bertke S., Dahm M.M., Yiin J.H., Kubale T.L., Hales T.R., Baris D., Zahm S.H., Beaumont J.J., Waters K.M., et al. Exposure–Response Relationships for Select Cancer and Non- Cancer Health Outcomes in a Cohort of US Firefighters from San Francisco, Chicago and Philadelphia (1950–2009) Occup Env. Med. 2015;72:699–706. doi: 10.1136/oemed-2014-102671. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

