Preclinical Safety Evaluation of Intranasally Delivered Human Mesenchymal Stem Cells in Juvenile Mice
- PMID: 33803160
- PMCID: PMC7963187
- DOI: 10.3390/cancers13051169
Preclinical Safety Evaluation of Intranasally Delivered Human Mesenchymal Stem Cells in Juvenile Mice
Abstract
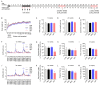
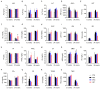
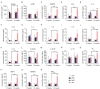
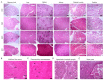
Mesenchymal stem cell (MSC)-based therapy is a promising therapeutic approach in the management of several pathologies, including central nervous system diseases. Previously, we demonstrated the therapeutic potential of human adipose-derived MSCs for neurological sequelae of oncological radiotherapy using the intranasal route as a non-invasive delivery method. However, a comprehensive investigation of the safety of intranasal MSC treatment should be performed before clinical applications. Here, we cultured human MSCs in compliance with quality control standards and administrated repeated doses of cells into the nostrils of juvenile immunodeficient mice, mimicking the design of a subsequent clinical trial. Short- and long-term effects of cell administration were evaluated by in vivo and ex vivo studies. No serious adverse events were reported on mouse welfare, behavioral performances, and blood plasma analysis. Magnetic resonance study and histological analysis did not reveal tumor formation or other abnormalities in the examined organs of mice receiving MSCs. Biodistribution study reveals a progressive disappearance of transplanted cells that was further supported by an absent expression of human GAPDH gene in the major organs of transplanted mice. Our data indicate that the intranasal application of MSCs is a safe, simple and non-invasive strategy and encourage its use in future clinical trials.
Keywords: biosafety; cell therapy; intranasal delivery; mesenchymal stem cells; nervous system disorders.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Rushkevich Y.N., Kosmacheva S.M., Zabrodets G.V., Ignatenko S.I., Goncharova N.V., Severin I.N., Likhachev S.A., Potapnev M.P. The Use of Autologous Mesenchymal Stem Cells for Cell Therapy of Patients with Amyotrophic Lateral Sclerosis in Belarus. Bull. Exp. Biol. Med. 2015;159:576–581. doi: 10.1007/s10517-015-3017-3. - DOI - PubMed
-
- Gotherstrom C., Westgren M., Shaw S.W., Astrom E., Biswas A., Byers P.H., Mattar C., Graham G., Taslimi J., Uwe U., et al. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: A two-center experience. Stem Cells Transl. Med. 2014;3:255–264. doi: 10.5966/sctm.2013-0090. - DOI - PMC - PubMed
-
- Vega A., Martin-Ferrero M.A., Del Canto F., Alberca M., Garcia V., Munar A., Orozco L., Soler R., Fuertes J., Huguet M., et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation. 2015;99:1681–1690. doi: 10.1097/TP.0000000000000678. - DOI - PubMed
-
- Connick P., Kolappan M., Crawley C., Webber D.J., Patani R., Michell A.W., Du M.-Q., Luan S.-L., Altmann D.R., Thompson A.J., et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. - DOI - PMC - PubMed
-
- Thakkar U.G., Trivedi H.L., Vanikar A.V., Dave S.D. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow–derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy. 2015;17:940–947. doi: 10.1016/j.jcyt.2015.03.608. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

