Nutrient-Induced Cellular Mechanisms of Gut Hormone Secretion
- PMID: 33803183
- PMCID: PMC8000029
- DOI: 10.3390/nu13030883
Nutrient-Induced Cellular Mechanisms of Gut Hormone Secretion
Abstract
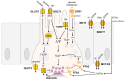
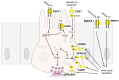
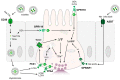
The gastrointestinal tract can assess the nutrient composition of ingested food. The nutrient-sensing mechanisms in specialised epithelial cells lining the gastrointestinal tract, the enteroendocrine cells, trigger the release of gut hormones that provide important local and central feedback signals to regulate nutrient utilisation and feeding behaviour. The evidence for nutrient-stimulated secretion of two of the most studied gut hormones, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), along with the known cellular mechanisms in enteroendocrine cells recruited by nutrients, will be the focus of this review. The mechanisms involved range from electrogenic transporters, ion channel modulation and nutrient-activated G-protein coupled receptors that converge on the release machinery controlling hormone secretion. Elucidation of these mechanisms will provide much needed insight into postprandial physiology and identify tractable dietary approaches to potentially manage nutrition and satiety by altering the secreted gut hormone profile.
Keywords: GIP; GLP-1; chemosensory; enteroendocrine cells; hormones; nutrients.
Conflict of interest statement
The FR/FMG lab receives additional grant support from AstraZeneca and Eli Lilly for unrelated work. FMG is a consultant for Kallyope (New York, NY, USA).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

