Soluble Fraction from Lysate of a High Concentration Multi-Strain Probiotic Formulation Inhibits TGF-β1-Induced Intestinal Fibrosis on CCD-18Co Cells
- PMID: 33803197
- PMCID: PMC7998462
- DOI: 10.3390/nu13030882
Soluble Fraction from Lysate of a High Concentration Multi-Strain Probiotic Formulation Inhibits TGF-β1-Induced Intestinal Fibrosis on CCD-18Co Cells
Abstract
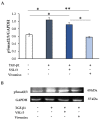
Fibrosis is a severe complication of chronic inflammatory disorders, such as inflammatory bowel disease (IBD). Current strategies are not fully effective in treating fibrosis; therefore, innovative anti-fibrotic approaches are urgently needed. TGF-β1 plays a central role in the fibrotic process by inducing myofibroblast differentiation and excessive extracellular matrix (ECM) protein deposition. Here, we explored the potential anti-fibrotic impact of two high concentration multi-strain probiotic formulations on TGF-β1-activated human intestinal colonic myofibroblast CCD-18Co. Human colonic fibroblast CCD-18Co cells were cultured in the presence of TGF-β1 to develop a fibrotic phenotype. Cell viability and growth were measured using the Trypan Blue dye exclusion test. The collagen-I, α-SMA, and pSmad2/3 expression levels were evaluated by Western blot analysis. Fibrosis markers were also analyzed by immunofluorescence and microscopy. The levels of TGF-β1 in the culture medium were assessed by ELISA. The effects of commercially available probiotic products VSL#3® and Vivomixx® were evaluated as the soluble fraction of bacterial lysates. The results suggested that the soluble fraction of Vivomixx® formulation, but not VSL#3®, was able to antagonize the pro-fibrotic effects of TGF-β1 on CCD-18Co cells, being able to prevent all of the cellular and molecular parameters that are related to the fibrotic phenotype. The mechanism underlying the observed effect appeared to be associated with inhibition of the TGF-β1/Smad signaling pathway. To our knowledge, this study provides the first experimental evidence that Vivomixx® could be considered to be a promising candidate against intestinal fibrosis, being able to antagonize TGF-β1 pro-fibrotic effects. The differences that were observed in our fibrosis model between the two probiotics used could be attributable to the different number of strains in different proportions.
Keywords: CCD-18Co cells; Smad 2/3; TGF-β1; VSL#3®; Vivomixx®; collagen-I; intestinal fibrosis; α-SMA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

