The Early Fragmentation of a Bovine Dermis-Derived Collagen Barrier Membrane Contributes to Transmembraneous Vascularization-A Possible Paradigm Shift for Guided Bone Regeneration
- PMID: 33803205
- PMCID: PMC7999168
- DOI: 10.3390/membranes11030185
The Early Fragmentation of a Bovine Dermis-Derived Collagen Barrier Membrane Contributes to Transmembraneous Vascularization-A Possible Paradigm Shift for Guided Bone Regeneration
Abstract
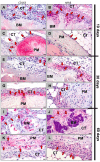
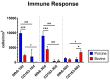
Collagen-based barrier membranes are an essential component in Guided Bone Regeneration (GBR) procedures. They act as cell-occlusive devices that should maintain a micromilieu where bone tissue can grow, which in turn provides a stable bed for prosthetic implantation. However, the standing time of collagen membranes has been a challenging area, as native membranes are often prematurely resorbed. Therefore, consolidation techniques, such as chemical cross-linking, have been used to enhance the structural integrity of the membranes, and by consequence, their standing time. However, these techniques have cytotoxic tendencies and can cause exaggerated inflammation and in turn, premature resorption, and material failures. However, tissues from different extraction sites and animals are variably cross-linked. For the present in vivo study, a new collagen membrane based on bovine dermis was extracted and compared to a commercially available porcine-sourced collagen membrane extracted from the pericardium. The membranes were implanted in Wistar rats for up to 60 days. The analyses included well-established histopathological and histomorphometrical methods, including histochemical and immunohistochemical staining procedures, to detect M1- and M2-macrophages as well as blood vessels. Initially, the results showed that both membranes remained intact up to day 30, while the bovine membrane was fragmented at day 60 with granulation tissue infiltrating the implantation beds. In contrast, the porcine membrane remained stable without signs of material-dependent inflammatory processes. Therefore, the bovine membrane showed a special integration pattern as the fragments were found to be overlapping, providing secondary porosity in combination with a transmembraneous vascularization. Altogether, the bovine membrane showed comparable results to the porcine control group in terms of biocompatibility and standing time. Moreover, blood vessels were found within the bovine membranes, which can potentially serve as an additional functionality of barrier membranes that conventional barrier membranes do not provide.
Keywords: Guided Bone Regeneration (GBR); barrier membrane; bovine collagen; bovine dermis; porcine collagen; porcine pericardium; tissue regeneration; tissue source; transmembraneous vascularization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ghanaati S. In vivo Implantation of a Bovine-Derived Collagen Membrane Leads to Changes in the Physiological Cellular Pattern of Wound Healing by the Induction of Multinucleated Giant Cells: An Adverse Reaction? Front. Bioeng. Biotechnol. 2018;6:1–13. doi: 10.3389/fbioe.2018.00104. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

