Expanding the Spectrum of Pancreatic Cancers Responsive to Vesicular Stomatitis Virus-Based Oncolytic Virotherapy: Challenges and Solutions
- PMID: 33803211
- PMCID: PMC7963195
- DOI: 10.3390/cancers13051171
Expanding the Spectrum of Pancreatic Cancers Responsive to Vesicular Stomatitis Virus-Based Oncolytic Virotherapy: Challenges and Solutions
Abstract
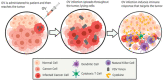
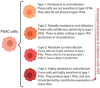
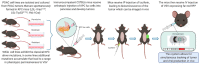
Pancreatic ductal adenocarcinoma (PDAC) is a devastating malignancy with poor prognosis and a dismal survival rate, expected to become the second leading cause of cancer-related deaths in the United States. Oncolytic virus (OV) is an anticancer approach that utilizes replication-competent viruses to preferentially infect and kill tumor cells. Vesicular stomatitis virus (VSV), one such OV, is already in several phase I clinical trials against different malignancies. VSV-based recombinant viruses are effective OVs against a majority of tested PDAC cell lines. However, some PDAC cell lines are resistant to VSV. Upregulated type I IFN signaling and constitutive expression of a subset of interferon-simulated genes (ISGs) play a major role in such resistance, while other mechanisms, such as inefficient viral attachment and resistance to VSV-mediated apoptosis, also play a role in some PDACs. Several alternative approaches have been shown to break the resistance of PDACs to VSV without compromising VSV oncoselectivity, including (i) combinations of VSV with JAK1/2 inhibitors (such as ruxolitinib); (ii) triple combinations of VSV with ruxolitinib and polycations improving both VSV replication and attachment; (iii) combinations of VSV with chemotherapeutic drugs (such as paclitaxel) arresting cells in the G2/M phase; (iv) arming VSV with p53 transgenes; (v) directed evolution approach producing more effective OVs. The latter study demonstrated impressive long-term genomic stability of complex VSV recombinants encoding large transgenes, supporting further clinical development of VSV as safe therapeutics for PDAC.
Keywords: oncolytic virus; pancreatic cancer; pancreatic ductal adenocarcinoma; vesicular stomatitis virus; virotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Ruxolitinib and Polycation Combination Treatment Overcomes Multiple Mechanisms of Resistance of Pancreatic Cancer Cells to Oncolytic Vesicular Stomatitis Virus.J Virol. 2017 Jul 27;91(16):e00461-17. doi: 10.1128/JVI.00461-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28566376 Free PMC article.
-
Experimental Evolution Generates Novel Oncolytic Vesicular Stomatitis Viruses with Improved Replication in Virus-Resistant Pancreatic Cancer Cells.J Virol. 2020 Jan 17;94(3):e01643-19. doi: 10.1128/JVI.01643-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694943 Free PMC article.
-
Intertumoral heterogeneity impacts oncolytic vesicular stomatitis virus efficacy in mouse pancreatic cancer cells.J Virol. 2023 Sep 28;97(9):e0100523. doi: 10.1128/jvi.01005-23. Epub 2023 Sep 6. J Virol. 2023. PMID: 37671865 Free PMC article.
-
Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer.J Gen Virol. 2012 Dec;93(Pt 12):2529-2545. doi: 10.1099/vir.0.046672-0. Epub 2012 Oct 10. J Gen Virol. 2012. PMID: 23052398 Free PMC article. Review.
-
Oncotargeting by Vesicular Stomatitis Virus (VSV): Advances in Cancer Therapy.Viruses. 2018 Feb 23;10(2):90. doi: 10.3390/v10020090. Viruses. 2018. PMID: 29473868 Free PMC article. Review.
Cited by
-
Exploiting RIG-I-like receptor pathway for cancer immunotherapy.J Hematol Oncol. 2023 Feb 8;16(1):8. doi: 10.1186/s13045-023-01405-9. J Hematol Oncol. 2023. PMID: 36755342 Free PMC article. Review.
-
Neutrophils in oncolytic virus immunotherapy.Front Immunol. 2024 Dec 4;15:1490414. doi: 10.3389/fimmu.2024.1490414. eCollection 2024. Front Immunol. 2024. PMID: 39697335 Free PMC article. Review.
-
Oncolytic Viruses in Ovarian Cancer: Where Do We Stand? A Narrative Review.Pathogens. 2025 Feb 3;14(2):140. doi: 10.3390/pathogens14020140. Pathogens. 2025. PMID: 40005517 Free PMC article. Review.
-
Role of the oral-gut microbiota axis in pancreatic cancer: a new perspective on tumor pathophysiology, diagnosis, and treatment.Mol Med. 2025 Mar 18;31(1):103. doi: 10.1186/s10020-025-01166-w. Mol Med. 2025. PMID: 40102723 Free PMC article. Review.
-
Exploiting Bacterial Effector Proteins to Uncover Evolutionarily Conserved Antiviral Host Machinery.bioRxiv [Preprint]. 2024 Jan 30:2024.01.29.577891. doi: 10.1101/2024.01.29.577891. bioRxiv. 2024. Update in: PLoS Pathog. 2024 May 16;20(5):e1012010. doi: 10.1371/journal.ppat.1012010. PMID: 38352400 Free PMC article. Updated. Preprint.
References
-
- Orth M., Metzger P., Gerum S., Mayerle J., Schneider G., Belka C., Schnurr M., Lauber K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019;14:1–20. doi: 10.1186/s13014-019-1345-6. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

