Recent Advances in Understanding the Role of Autophagy in Paediatric Brain Tumours
- PMID: 33803216
- PMCID: PMC8000899
- DOI: 10.3390/diagnostics11030481
Recent Advances in Understanding the Role of Autophagy in Paediatric Brain Tumours
Abstract
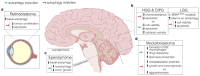
Autophagy is a degradative process occurring in eukaryotic cells to maintain homeostasis and cell survival. After stressful conditions including nutrient deprivation, hypoxia or drugs administration, autophagy is induced to counteract pathways that could lead to cell death. In cancer, autophagy plays a paradoxical role, acting both as tumour suppressor-by cleaning cells from damaged organelles and inhibiting inflammation or, alternatively, by promoting genomic stability and tumour adaptive response-or as a pro-survival mechanism to protect cells from stresses such as chemotherapy. Neural-derived paediatric solid tumours represent a variety of childhood cancers with unique anatomical location, cellular origins, and clinical presentation. These tumours are a leading cause of morbidity and mortality among children and new molecular diagnostics and therapies are necessary for longer survival and reduced morbidity. Here, we review advances in our understanding of how autophagy modulation exhibits antitumor properties in experimental models of paediatric brain tumours, i.e., medulloblastoma (MB), ependymoma (EPN), paediatric low-grade and high-grade gliomas (LGGs, HGGs), atypical teratoid/rhabdoid tumours (ATRTs), and retinoblastoma (RB). We also discuss clinical perspectives to consider how targeting autophagy may be relevant in these specific paediatric tumours.
Keywords: autophagy; brain tumours; oncology; targeted therapy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

