Neuroimaging Correlates of Cognitive Dysfunction in Adults with Multiple Sclerosis
- PMID: 33803287
- PMCID: PMC8000635
- DOI: 10.3390/brainsci11030346
Neuroimaging Correlates of Cognitive Dysfunction in Adults with Multiple Sclerosis
Abstract
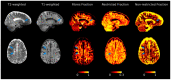
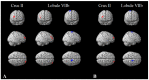
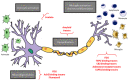
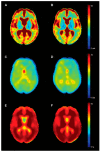
Cognitive impairment is a frequent and meaningful symptom in multiple sclerosis (MS), caused by the accrual of brain structural damage only partially counteracted by effective functional reorganization. As both these aspects can be successfully investigated through the application of advanced neuroimaging, here, we offer an up-to-date overview of the latest findings on structural, functional and metabolic correlates of cognitive impairment in adults with MS, focusing on the mechanisms sustaining damage accrual and on the identification of useful imaging markers of cognitive decline.
Keywords: atrophy; cognitive dysfunction; magnetic resonance imaging; multiple sclerosis; neuroimaging; positron emission tomography; sodium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Uher T., Blahova-Dusankova J., Horakova D., Bergsland N., Tyblova M., Benedict R.H., Kalincik T., Ramasamy D.P., Seidl Z., Hagermeier J., et al. Longitudinal MRI and neuropsychological assessment of patients with clinically isolated syndrome. J. Neurol. 2014;261:1735–1744. doi: 10.1007/s00415-014-7413-9. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

