Exatecan Antibody Drug Conjugates Based on a Hydrophilic Polysarcosine Drug-Linker Platform
- PMID: 33803327
- PMCID: PMC8000490
- DOI: 10.3390/ph14030247
Exatecan Antibody Drug Conjugates Based on a Hydrophilic Polysarcosine Drug-Linker Platform
Abstract
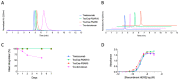
We herein report the development and evaluation of a novel HER2-targeting antibody-drug conjugate (ADC) based on the topoisomerase I inhibitor payload exatecan, using our hydrophilic monodisperse polysarcosine (PSAR) drug-linker platform (PSARlink). In vitro and in vivo experiments were conducted in breast and gastric cancer models to characterize this original ADC and gain insight about the drug-linker structure-activity relationship. The inclusion of the PSAR hydrophobicity masking entity efficiently reduced the overall hydrophobicity of the conjugate and yielded an ADC sharing the same pharmacokinetic profile as the unconjugated antibody despite the high drug-load of the camptothecin-derived payload (drug-antibody ratio of 8). Tra-Exa-PSAR10 demonstrated strong anti-tumor activity at 1 mg/kg in an NCI-N87 xenograft model, outperforming the FDA-approved ADC DS-8201a (Enhertu), while being well tolerated in mice at a dose of 100 mg/kg. In vitro experiments showed that this exatecan-based ADC demonstrated higher bystander killing effect than DS-8201a and overcame resistance to T-DM1 (Kadcyla) in preclinical HER2+ breast and esophageal models, suggesting potential activity in heterogeneous and resistant tumors. In summary, the polysarcosine-based hydrophobicity masking approach allowsfor the generation of highly conjugated exatecan-based ADCs having excellent physicochemical properties, an improved pharmacokinetic profile, and potent in vivo anti-tumor activity.
Keywords: antibody–drug conjugates; camptothecin; deruxtecan; polysarcosine; topoisomerase I inhibitor.
Conflict of interest statement
L.C. and A.M. are employees of Mablink Bioscience. W.V., B.J. and C.D. are shareholders of Mablink Bioscience.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

