Integrin α10-Antibodies Reduce Glioblastoma Tumor Growth and Cell Migration
- PMID: 33803359
- PMCID: PMC7980568
- DOI: 10.3390/cancers13051184
Integrin α10-Antibodies Reduce Glioblastoma Tumor Growth and Cell Migration
Abstract
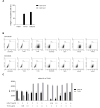
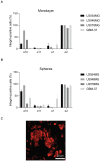
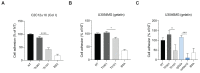
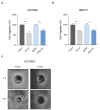
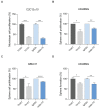
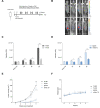
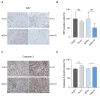
Glioblastoma (GB) is the most common and the most aggressive form of brain tumor in adults, which currently lacks efficient treatment strategies. In this study, we investigated the therapeutic effect of function-blocking antibodies targeting integrin α10β1 on patient-derived-GB cell lines in vitro and in vivo. The in vitro studies demonstrated significant inhibiting effects of the integrin α10 antibodies on the adhesion, migration, proliferation, and sphere formation of GB cells. In a xenograft mouse model, the effect of the antibodies on tumor growth was investigated in luciferase-labeled and subcutaneously implanted GB cells. As demonstrated by in vivo imaging analysis and caliper measurements, the integrin α10-antibodies significantly suppressed GB tumor growth compared to control antibodies. Immunohistochemical analysis of the GB tumors showed lower expression of the proliferation marker Ki67 and an increased expression of cleaved caspase-3 after treatment with integrin α10 antibodies, further supporting a therapeutic effect. Our results suggest that function-blocking antibody targeting integrin α10β1 is a promising therapeutic strategy for the treatment of glioblastoma.
Keywords: collagen; function-blocking antibody; glioblastoma; integrin α10β1; migration; tumor growth.
Conflict of interest statement
E.L.-Å., K.C.M., and X.H. own shares in Xintela AB; K.C.M., X.H., and E.L.-Å. are employed by Xintela AB. The other authors declare no conflicts of interest.
Figures
Similar articles
-
Integrin α10, a Novel Therapeutic Target in Glioblastoma, Regulates Cell Migration, Proliferation, and Survival.Cancers (Basel). 2019 Apr 25;11(4):587. doi: 10.3390/cancers11040587. Cancers (Basel). 2019. PMID: 31027305 Free PMC article.
-
Integrin α10β1-Selected Mesenchymal Stem Cells Mitigate the Progression of Osteoarthritis in an Equine Talar Impact Model.Am J Sports Med. 2020 Mar;48(3):612-623. doi: 10.1177/0363546519899087. Epub 2020 Jan 31. Am J Sports Med. 2020. PMID: 32004077
-
Distribution of the collagen-binding integrin alpha10beta1 during mouse development.Cell Tissue Res. 2001 Oct;306(1):107-16. doi: 10.1007/s004410100385. Cell Tissue Res. 2001. PMID: 11683172
-
Integrin α10β1: a collagen receptor critical in skeletal development.Adv Exp Med Biol. 2014;819:61-71. doi: 10.1007/978-94-017-9153-3_4. Adv Exp Med Biol. 2014. PMID: 25023167 Review.
-
CAR-Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors Into Precision Tools for Cancer Immunotherapy.Front Immunol. 2019 Nov 14;10:2683. doi: 10.3389/fimmu.2019.02683. eCollection 2019. Front Immunol. 2019. PMID: 31798595 Free PMC article. Review.
Cited by
-
Glucocorticoid treatment influences prostate cancer cell growth and the tumor microenvironment via altered glucocorticoid receptor signaling in prostate fibroblasts.Oncogene. 2024 Jan;43(4):235-247. doi: 10.1038/s41388-023-02901-5. Epub 2023 Nov 29. Oncogene. 2024. PMID: 38017134 Free PMC article.
-
Inhibitory Effects of Urolithins, Bioactive Gut Metabolites from Natural Polyphenols, against Glioblastoma Progression.Nutrients. 2023 Nov 21;15(23):4854. doi: 10.3390/nu15234854. Nutrients. 2023. PMID: 38068712 Free PMC article.
-
Perphenazine and prochlorperazine decrease glioblastoma U-87 MG cell migration and invasion: Analysis of the ABCB1 and ABCG2 transporters, E-cadherin, α-tubulin and integrins (α3, α5, and β1) levels.Oncol Lett. 2022 Jun;23(6):182. doi: 10.3892/ol.2022.13302. Epub 2022 Apr 15. Oncol Lett. 2022. PMID: 35527777 Free PMC article.
-
The characteristics and the multiple functions of integrin β1 in human cancers.J Transl Med. 2023 Nov 6;21(1):787. doi: 10.1186/s12967-023-04696-1. J Transl Med. 2023. PMID: 37932738 Free PMC article. Review.
-
Collagen remodeling-mediated signaling pathways and their impact on tumor therapy.J Biol Chem. 2025 Mar;301(3):108330. doi: 10.1016/j.jbc.2025.108330. Epub 2025 Feb 19. J Biol Chem. 2025. PMID: 39984051 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

