Stress and Nasal Allergy: Corticotropin-Releasing Hormone Stimulates Mast Cell Degranulation and Proliferation in Human Nasal Mucosa
- PMID: 33803422
- PMCID: PMC7967145
- DOI: 10.3390/ijms22052773
Stress and Nasal Allergy: Corticotropin-Releasing Hormone Stimulates Mast Cell Degranulation and Proliferation in Human Nasal Mucosa
Abstract
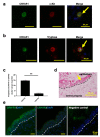
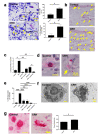
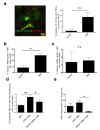
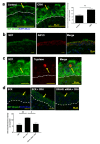
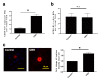
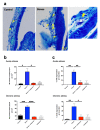
Psychological stress exacerbates mast cell (MC)-dependent inflammation, including nasal allergy, but the underlying mechanisms are not thoroughly understood. Because the key stress-mediating neurohormone, corticotropin-releasing hormone (CRH), induces human skin MC degranulation, we hypothesized that CRH may be a key player in stress-aggravated nasal allergy. In the current study, we probed this hypothesis in human nasal mucosa MCs (hM-MCs) in situ using nasal polyp organ culture and tested whether CRH is required for murine M-MC activation by perceived stress in vivo. CRH stimulation significantly increased the number of hM-MCs, stimulated both their degranulation and proliferation ex vivo, and increased stem cell factor (SCF) expression in human nasal mucosa epithelium. CRH also sensitized hM-MCs to further CRH stimulation and promoted a pro-inflammatory hM-MC phenotype. The CRH-induced increase in hM-MCs was mitigated by co-administration of CRH receptor type 1 (CRH-R1)-specific antagonist antalarmin, CRH-R1 small interfering RNA (siRNA), or SCF-neutralizing antibody. In vivo, restraint stress significantly increased the number and degranulation of murine M-MCs compared with sham-stressed mice. This effect was mitigated by intranasal antalarmin. Our data suggest that CRH is a major activator of hM-MC in nasal mucosa, in part via promoting SCF production, and that CRH-R1 antagonists such as antalarmin are promising candidate therapeutics for nasal mucosa neuroinflammation induced by perceived stress.
Keywords: CRH; mast cell; nasal mucosa; psychological stress; stem cell factor (SCF).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Philpott C., Erskine S., Hopkins C., Coombes E., Kara N., Sunkareneni V., Anari S., Salam M., Farboud A., Clark A. A case-control study of medical, psychological and socio-economic factors influencing the severity of chronic rhinosinusitis. Rhinology. 2016;54:134–140. doi: 10.4193/Rhin15.272. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

