Synthesis and Pharmacological In Vitro Investigations of Novel Shikonin Derivatives with a Special Focus on Cyclopropane Bearing Derivatives
- PMID: 33803437
- PMCID: PMC7967198
- DOI: 10.3390/ijms22052774
Synthesis and Pharmacological In Vitro Investigations of Novel Shikonin Derivatives with a Special Focus on Cyclopropane Bearing Derivatives
Abstract
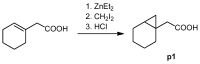
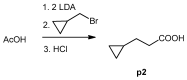
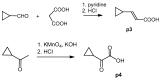
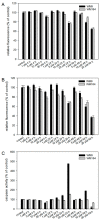
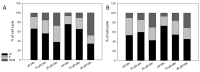
Melanoma is the deadliest form of skin cancer and accounts for about three quarters of all skin cancer deaths. Especially at an advanced stage, its treatment is challenging, and survival rates are very low. In previous studies, we showed that the constituents of the roots of Onosma paniculata as well as a synthetic derivative of the most active constituent showed promising results in metastatic melanoma cell lines. In the current study, we address the question whether we can generate further derivatives with optimized activity by synthesis. Therefore, we prepared 31, mainly novel shikonin derivatives and screened them in different melanoma cell lines (WM9, WM164, and MUG-Mel2 cells) using the XTT viability assay. We identified (R)-1-(1,4-dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-cyclopropyl-2-oxoacetate as a novel derivative with even higher activity. Furthermore, pharmacological investigations including the ApoToxGloTM Triplex assay, LDH assay, and cell cycle measurements revealed that this compound induced apoptosis and reduced cells in the G1 phase accompanied by an increase of cells in the G2/M phase. Moreover, it showed hardly any effects on the cell membrane integrity. However, it also exhibited cytotoxicity against non-tumorigenic cells. Nevertheless, in summary, we could show that shikonin derivatives might be promising drug leads in the treatment of melanoma.
Keywords: apoptosis; cell cycle; melanoma; shikonin derivatives; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
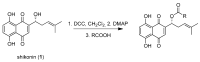


References
-
- Gershenwald J.E., Scolyer R.A., Hess K.R., Sondak V.K., Long G.V., Ross M.I., Lazar A.J., Faries M.B., Kirkwood J.M., McArthur G.A., et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017;67:472–492. doi: 10.3322/caac.21409. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

