Microglial Heterogeneity and Its Potential Role in Driving Phenotypic Diversity of Alzheimer's Disease
- PMID: 33803478
- PMCID: PMC7967159
- DOI: 10.3390/ijms22052780
Microglial Heterogeneity and Its Potential Role in Driving Phenotypic Diversity of Alzheimer's Disease
Abstract
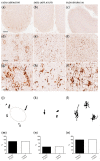
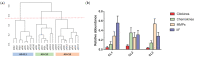
Alzheimer's disease (AD) is increasingly recognized as a highly heterogeneous disorder occurring under distinct clinical and neuropathological phenotypes. Despite the molecular determinants of such variability not being well defined yet, microglial cells may play a key role in this process by releasing distinct pro- and/or anti-inflammatory cytokines, potentially affecting the expression of the disease. We carried out a neuropathological and biochemical analysis on a series of AD brain samples, gathering evidence about the heterogeneous involvement of microglia in AD. The neuropathological studies showed differences concerning morphology, density and distribution of microglial cells among AD brains. Biochemical investigations showed increased brain levels of IL-4, IL-6, IL-13, CCL17, MMP-7 and CXCL13 in AD in comparison with control subjects. The molecular profiling achieved by measuring the brain levels of 25 inflammatory factors known to be involved in neuroinflammation allowed a stratification of the AD patients in three distinct "neuroinflammatory clusters". These findings strengthen the relevance of neuroinflammation in AD pathogenesis suggesting, in particular, that the differential involvement of neuroinflammatory molecules released by microglial cells during the development of the disease may contribute to modulate the characteristics and the severity of the neuropathological changes, driving-at least in part-the AD phenotypic diversity.
Keywords: Alzheimer’s disease; Aβ; MMPs; chemokines; cytokines; dementia; heterogeneity; innate immunity factors; microglia; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Morris J.C. Early-stage and preclinical Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2005;19:163–165. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

