Protective Effects of Taurine Chloramine on Experimentally Induced Colitis: NFκB, STAT3, and Nrf2 as Potential Targets
- PMID: 33803551
- PMCID: PMC8002934
- DOI: 10.3390/antiox10030479
Protective Effects of Taurine Chloramine on Experimentally Induced Colitis: NFκB, STAT3, and Nrf2 as Potential Targets
Abstract
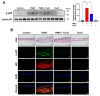
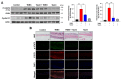
Taurine chloramine (TauCl) is an endogenous anti-inflammatory substance which is derived from taurine, a semi-essential sulfur-containing β-amino acid found in some foods including meat, fish, eggs and milk. In general, TauCl as well as its parent compound taurine downregulates production of tissue-damaging proinflammatory mediators, such as chemokines and cytokines in many different types of cells. In the present study, we investigated the protective effects of TauCl on experimentally induced colon inflammation. Oral administration of TauCl protected against mouse colitis caused by 2,4,6-trinitrobenzene sulfonic acid (TNBS). TauCl administration attenuated apoptosis in the colonic mucosa of TNBS-treated mice. This was accompanied by reduced expression of an oxidative stress marker, 4-hydroxy-2-nonenal and proinflammatory molecules including tumor necrosis factor-α, interleukin-6 and cyclooxygenase-2 in mouse colon. TauCl also inhibited activation of NFκB and STAT3, two key transcription factors mediating proinflammatory signaling. Notably, the protective effect of TauCl on oxidative stress and inflammation in the colon of TNBS-treated mice was associated with elevated activation of Nrf2 and upregulation of its target genes encoding heme oxygenase-1, NAD(P)H:quinone oxidoreductase, glutamate cysteine ligase catalytic subunit, and glutathione S-transferase. Taken together, these results suggest that TauCl exerts the protective effect against colitis through upregulation of Nrf2-dependent cytoprotective gene expression while blocking the proinflammatory signaling mediated by NFκB and STAT3.
Keywords: 2,4,6-trinitrobenzene sulfonic acid; NFκB; Nrf2; STAT3; colitis; heme oxygenase-1; taurine; taurine chloramine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Centers for Disease Control and Prevention What Is Inflammatory Bowel Disease (IBD)? [(accessed on 12 February 2021)];2018 Available online: https://www.cdc.gov/ibd/what-is-IBD.htm.
-
- Luceri C., Bigagli E., Agostiniani S., Giudici F., Zambonin D., Scaringi S., Ficari F., Lodovici M., Malentacchi C. Analysis of oxidative stress-related markers in crohn’s disease patients at surgery and correlations with clinical findings. Antioxidants. 2019;8:378. doi: 10.3390/antiox8090378. - DOI - PMC - PubMed
-
- Alemany-Cosme E., Sáez-González E., Moret I., Mateos B., Iborra M., Nos P., Sandoval J., Beltrán B. Oxidative stress in the pathogenesis of Crohn’s disease and the interconnection with Immuiological response, microbiota, external environmental factors, and epigenetics. Antioxidants. 2021;10:64. doi: 10.3390/antiox10010064. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

