External Validation and Extension of a Clinical Score for the Discrimination of Type 2 Myocardial Infarction
- PMID: 33803801
- PMCID: PMC8003225
- DOI: 10.3390/jcm10061264
External Validation and Extension of a Clinical Score for the Discrimination of Type 2 Myocardial Infarction
Abstract
Background: The early non-invasive discrimination of Type 2 versus Type 1 Myocardial Infarction (T2MI, T1MI) is a major unmet clinical need. We aimed to externally validate a recently derived clinical score (Neumann) combing female sex, no radiating chest pain, and high-sensitivity cardiac troponin I (hs-cTnI) concentration ≤40.8 ng/L.
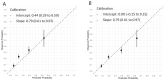
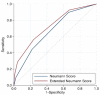
Methods: Patients presenting with acute chest discomfort to the emergency department were prospectively enrolled into an international multicenter diagnostic study. The final diagnoses of T2MI and T1MI were centrally adjudicated by two independent cardiologists using all information including cardiac imaging and serial measurements of hs-cTnT/I according to the fourth universal definition of MI. Model performance for T2MI diagnosis was assessed by formal tests and graphical means of discrimination and calibration.
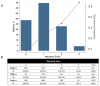
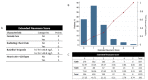
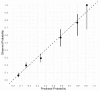
Results: Among 6684 enrolled patients, MI was the adjudicated final diagnosis in 1079 (19%) patients, of which 242 (22%) had T2MI. External validation of the Neumann Score showed a moderate discrimination (C-statistic 0.67 (95%CI 0.64-0.71)). Model calibration showed underestimation of the predicted probabilities of having T2MI for low point scores. Model extension by adding the binary variable heart rate >120/min significantly improved model performance (C-statistic 0.73 (95% CI 0.70-0.76, p < 0.001) and had good calibration. Patients with the highest score values of 3 (Neumann Score, 9.9%) and 5 (Extended Neumann Score, 3.3%) had a 53% and 91% predicted probability of T2MI, respectively.
Conclusion: The Neumann Score provided moderate discrimination and suboptimal calibration. Extending the Neumann Score by adding heart rate >120/min improved the model's performance.
Keywords: differentiation; external validation; risk scores; type 1 myocardial infarction; type 2 myocardial infarction.
Conflict of interest statement
We disclose that Nestelberger has received research support from the Swiss National Science Foundation (P400PM_191037/1), the Swiss Heart Foundation (FF20079), the Max Cloëtta Foundation, the Margarete und Walter Lichtenstein-Stiftung (3MS1038), and the University Hospital Basel as well as speaker honoraria/consulting honoraria from B.Braun, Siemens, Beckman Coulter, Bayer, Ortho Clinical Diagnostics, and Orion Pharma, outside the submitted work. Lopez-Ayala has received research funding from the Swiss Heart Foundation (FF20079), outside the submitted work. Boeddinghaus has received research grants from the University of Basel and the Division of Internal Medicine, the Swiss Academy of Medical Sciences, the Gottfried and Julia Bangerter-Rhyner-Foundation, and speaker honoraria from Siemens, outside the submitted work. Rubini Gimenez has received research grants from the Swiss Heart Foundation and Swiss National Science Foundation (P400PM_180828) as well as speakers/consulting honoraria from Abbott, Ortho Clinical Diagnostics, Roche, and Siemens, outside the submitted work. Wildi has received research funding from the FAG Basel, and the Julia und Gottfried Bangerter-Rhyner Stiftung, the Prince Charles Hospital Foundation, the CRE Action Fund (NHMRC), the Wesley Medical Research Foundation, and a PhD n UQ scholarship from the University of Queensland, all outside the submitted work. Koechlin has received a research grant from the University of Basel, the Swiss Academy of Medical Sciences, and the Gottfried and Julia Bangerter-Rhyner Foundation, as well as the “Freiwillige Akademische Gesellschaft Basel”, outside the submitted work. Gualandro has received consulting honoraria from Roche, outside the submitted work. Martin-Sanchez has received speaker, advisory or consulting fees from Novartis, MSD, Bristol–Myers Squibb, Pfizer, The Medicine Company, Otsuka, Thermo Fisher, Cardiorentis, Sanofi, and research grants from the Spanish Ministry of Health and FEDER, Mapfre, Novartis, Bayer, MSD, Abbott, and Orion-Pharma, outside the submitted work. Twerenbold has received research support from the Swiss National Science Foundation (P300PB-167803/1) and speaker honoraria/consulting honoraria from Roche, Abbott, Brahms and Siemens, outside the submitted work. Mueller has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the Stiftung für kardiovaskuläre Forschung Basel; Abbott, Alere, Astra Zeneca, Beckman Coulter, Biomerieux, Brahms, Roche, Siemens, Singulex, Sphingotec, and the Department of Internal Medicine, University Hospital Basel, as well as speaker honoraria/consulting honoraria from Abbott, Alere, Astra Zeneca, Biomerieux, Boehringer Ingelheim, BMS, Brahms, Cardiorentis, Novartis, Roche, Siemens, and Singulex, outside the submitted work. All other authors declare that they have no conflict of interest with this study. The investigated hs-cTn assay were donated by the manufacturer, who had no role in the design of the study, the analysis of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
Figures


References
-
- Collet J.-P., Thiele H., Barbato E., Barthélémy O., Bauersachs J., Bhatt D.L., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Hear. J. 2020 doi: 10.1093/eurheartj/ehaa624. - DOI - PubMed
-
- Nestelberger T., Boeddinghaus J., Badertscher P., Twerenbold R., Wildi K., Breitenbücher D., Sabti Z., Puelacher C., Giménez M.R., Kozhuharov N., et al. Effect of Definition on Incidence and Prognosis of Type 2 Myocardial Infarction. J. Am. Coll. Cardiol. 2017;70:1558–1568. doi: 10.1016/j.jacc.2017.07.774. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

