Identification of Genetic Modifiers of TDP-43: Inflammatory Activation of Astrocytes for Neuroinflammation
- PMID: 33803845
- PMCID: PMC8003223
- DOI: 10.3390/cells10030676
Identification of Genetic Modifiers of TDP-43: Inflammatory Activation of Astrocytes for Neuroinflammation
Abstract
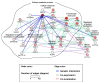
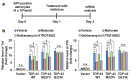
Transactive response DNA-binding protein 43 (TDP-43) is a ubiquitously expressed DNA/RNA-binding protein linked to amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). TDP-43 has been implicated in numerous aspects of the mRNA life cycle, as well as in cell toxicity and neuroinflammation. In this study, we used the toxicity of the TDP-43 expression in Saccharomyces cerevisiae as an assay to identify TDP-43 genetic interactions. Specifically, we transformed human TDP-43 cDNAs of wild-type or disease-associated mutants (M337V and Q331K) en masse into 4653 homozygous diploid yeast deletion mutants and then used next-generation sequencing readouts of growth to identify yeast toxicity modifiers. Genetic interaction analysis provided a global view of TDP-43 pathways, some of which are known to be involved in cellular metabolic processes. Selected putative loci with the potential of genetic interactions with TDP-43 were assessed for associations with neurotoxicity and inflammatory activation of astrocytes. The pharmacological inhibition of succinate dehydrogenase flavoprotein subunit A (SDHA) and voltage-dependent anion-selective channel 3 (VDAC3) suppressed TDP-43-induced expression of proinflammatory cytokines in astrocytes, indicating the critical roles played by SDHA and VDAC3 in TDP-43 pathways during inflammatory activation of astrocytes and neuroinflammation. Thus, the findings of our TDP-43 genetic interaction screen provide a global landscape of TDP-43 pathways and may help improve our understanding of the roles of glia and neuroinflammation in ALS and FTD pathogenesis.
Keywords: TDP-43; amyotrophic lateral sclerosis; astrocyte; genetic interaction; glia; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Xu Y.F., Gendron T.F., Zhang Y.J., Lin W.L., D’Alton S., Sheng H., Casey M.C., Tong J., Knight J., Yu X., et al. Wild-Type Human TDP-43 Expression Causes TDP-43 Phosphorylation, Mitochondrial Aggregation, Motor Deficits, and Early Mortality in Transgenic Mice. J. Neurosci. 2010;30:10851–10859. doi: 10.1523/JNEUROSCI.1630-10.2010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

