Petasis vs. Strecker Amino Acid Synthesis: Convergence, Divergence and Opportunities in Organic Synthesis
- PMID: 33803879
- PMCID: PMC8003338
- DOI: 10.3390/molecules26061707
Petasis vs. Strecker Amino Acid Synthesis: Convergence, Divergence and Opportunities in Organic Synthesis
Abstract
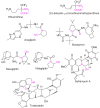
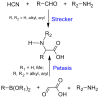
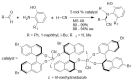
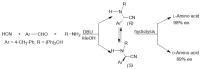
α-Amino acids find widespread applications in various areas of life and physical sciences. Their syntheses are carried out by a multitude of protocols, of which Petasis and Strecker reactions have emerged as the most straightforward and most widely used. Both reactions are three-component reactions using the same starting materials, except the nucleophilic species. The differences and similarities between these two important reactions are highlighted in this review.
Keywords: Petasis; Strecker; amino acids; biomolecules; multicomponent reactions; peptides.
Conflict of interest statement
The author declares no conflict of interest.
Figures

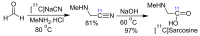

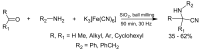
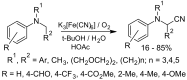
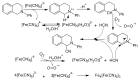
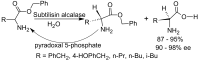

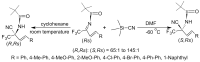

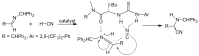
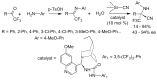
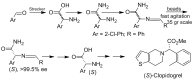
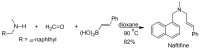

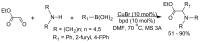
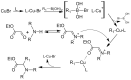




References
-
- Bada J. Strecker Synthesis. In: Gargaud M., Amils R., Quintanilla J.C., Cleaves H.J. II, Irvine W.M., Pinti D.L., Viso M., editors. Encyclopedia of Astrobiology. Springer; Berlin/Heidelberg, Germany: 2011. p. 1603. - DOI
-
- Strecker A. Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Justus Liebigs Ann. Der Chem. 1850;75:27–45. doi: 10.1002/jlac.18500750103. - DOI
-
- Strecker A. Ueber einen neuen aus Aldehyd-Ammoniak und Blausäure entstehenden Körper. Justus Liebigs Ann. Der Chem. 1854;91:349–351. doi: 10.1002/jlac.18540910309. - DOI
-
- Pascal R. Bücherer–Bergs Synthesis. In: Gargaud M., Amils R., Quintanilla J.C., Cleaves H.J. II, Irvine W.M., Pinti D.L., Viso M., editors. Encyclopedia of Astrobiology. Springer; Berlin/Heidelberg, Germany: 2011. pp. 221–222. - DOI
-
- Monteiro J.L., Pieber B., Corrêa A.G., Kappe C.O. Continuous synthesis of hydantoins: Intensifying the Bucherer–Bergs reaction. Synlett. 2016;27:83–87. doi: 10.1055/s-0035-1560317. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

