LAG-3-Expressing Tumor-Infiltrating T Cells Are Associated with Reduced Disease-Free Survival in Pancreatic Cancer
- PMID: 33803936
- PMCID: PMC7998134
- DOI: 10.3390/cancers13061297
LAG-3-Expressing Tumor-Infiltrating T Cells Are Associated with Reduced Disease-Free Survival in Pancreatic Cancer
Abstract
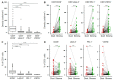
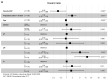
T cells are the predominant immune cell population in the pancreatic tumor microenvironment. High CD8+ and Th1-polarized CD4+ T cell infiltration is associated with prolonged survival in human pancreatic ductal adenocarcinoma (PDAC). However, the expression pattern of co-stimulatory and inhibitory receptors by PDAC-infiltrating T cells and their prognostic significance are not well defined. In this study, we employed multiplex immunofluorescence to investigate the intratumoral expression of the co-stimulatory receptor inducible T-cell co-stimulator (ICOS), the inhibitory receptors lymphocyte-activation gene 3 (LAG-3), programmed death 1 (PD-1), and V-domain immunoglobulin suppressor of T cell activation (VISTA) by tumor-infiltrating T cells (CD3) in a cohort of 69 patients with resected PDAC. T cells were enriched particularly within the stromal area and were highly heterogeneous across tumors. Further, T cells were associated with prolonged disease-free survival (DFS). However, LAG-3 expression by PDAC-infiltrating T cells was correlated with reduced DFS. Our study highlights the biological importance of LAG-3 expression by tumor-infiltrating T cells. LAG-3+ T cells may represent a novel prognostic marker and a particularly attractive target for immunotherapeutic strategies in PDAC.
Keywords: ICOS; LAG-3; PD-1; VISTA; pancreatic cancer; tumor microenvironment; tumor-infiltrating T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Royal R.E., Levy C., Turner K., Mathur A., Hughes M., Kammula U.S., Sherry R.M., Topalian S.L., Yang J.C., Lowy I., et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. - DOI - PMC - PubMed
-
- Patnaik A., Kang S.P., Rasco D., Papadopoulos K.P., Elassaiss-Schaap J., Beeram M., Drengler R., Chen C., Smith L., Espino G., et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015;21:4286–4293. doi: 10.1158/1078-0432.CCR-14-2607. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

