Antenna Protein Clustering In Vitro Unveiled by Fluorescence Correlation Spectroscopy
- PMID: 33804002
- PMCID: PMC8000295
- DOI: 10.3390/ijms22062969
Antenna Protein Clustering In Vitro Unveiled by Fluorescence Correlation Spectroscopy
Abstract
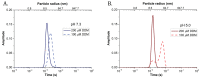
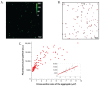
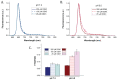
Antenna protein aggregation is one of the principal mechanisms considered effective in protecting phototrophs against high light damage. Commonly, it is induced, in vitro, by decreasing detergent concentration and pH of a solution of purified antennas; the resulting reduction in fluorescence emission is considered to be representative of non-photochemical quenching in vivo. However, little is known about the actual size and organization of antenna particles formed by this means, and hence the physiological relevance of this experimental approach is questionable. Here, a quasi-single molecule method, fluorescence correlation spectroscopy (FCS), was applied during in vitro quenching of LHCII trimers from higher plants for a parallel estimation of particle size, fluorescence, and antenna cluster homogeneity in a single measurement. FCS revealed that, below detergent critical micelle concentration, low pH promoted the formation of large protein oligomers of sizes up to micrometers, and therefore is apparently incompatible with thylakoid membranes. In contrast, LHCII clusters formed at high pH were smaller and homogenous, and yet still capable of efficient quenching. The results altogether set the physiological validity limits of in vitro quenching experiments. Our data also support the idea that the small, moderately quenching LHCII oligomers found at high pH could be relevant with respect to non-photochemical quenching in vivo.
Keywords: antenna proteins; detergent critical micelle concentration; fluorescence correlation spectroscopy; non-photochemical quenching; photoprotection; photosynthesis; protein oligomerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kana R., Vass I. Thermoimaging as a tool for studying light-induced heating of leaves Correlation of heat dissipation with the efficiency of photosystem II photochemistry and non-photochemical quenching. Environ. Exp. Bot. 2008;64:90–96. doi: 10.1016/j.envexpbot.2008.02.006. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

