Nephrogenic Systemic Fibrosis as a Complication after Gadolinium-Containing Contrast Agents: A Rapid Review
- PMID: 33804005
- PMCID: PMC8001337
- DOI: 10.3390/ijerph18063000
Nephrogenic Systemic Fibrosis as a Complication after Gadolinium-Containing Contrast Agents: A Rapid Review
Abstract
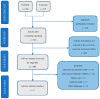
Introduction: Nephrogenic systemic fibrosis (NFS) is a generalized disorder occurring in people with kidney failure. This new disease entity can lead to significant disability or even death. Gadolinium-associated systemic fibrosis is related to exposure to contrast agents used for magnetic resonance imaging. The aim of this study was to review the literature in available scientific databases on NFS-complication after gadolinium-containing contrast agents. Methods: PubMed and Cochrane Library databases were searched using adequate key words. A literature review of the described cases of NSF occurrence after exposure to gadolinium-containing contrast agents was performed. A review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A review written protocol was not drafted. Results: Originally, 647 studies were searched in scientific databases. After rejecting the duplicate results, 515 results were obtained. Finally, nine studies were included in the review. A total of 173 cases with NSF were included in the analysis. The majority of patients were undergoing dialysis. The contrast agent used for MRI was most often gadodiamide and gadopentetate dimeglumine. The time from exposure to NSF symptoms was from two days to three years. Three authors pointed out other factors in their papers that could potentially influence the occurrence of NSF. These included: metabolic acidosis, ongoing infection, higher doses of erythropoietin and higher serum concentrations of ionized calcium and phosphate. Since 2008, the number of reported cases of NSF has decreased significantly. More recent guidelines and reports indicate that not all contrast agents are associated with the same risk of developing NSF. Conclusions: Most NSF occurs after exposure to linear contrast agents. Therefore, it is recommended to limit their use, especially in dialyzed patients and patients with a GFR < 30 mL/min.
Keywords: gadolinium; nephrogenic systemic fibrosis; renal failure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources