Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care
- PMID: 33804015
- PMCID: PMC8000881
- DOI: 10.3390/nu13030942
Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care
Abstract
Background: No study has explored the limitations of current long-term management of hyperkalemia (HK) in outpatient CKD clinics.
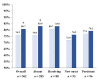
Methods: We evaluated the association between current therapeutic options and control of serum K (sK) during 12-month follow up in ND-CKD patients stratified in four groups by HK (sK ≥ 5.0 mEq/L) at baseline and month 12: Absent (no-no), Resolving (yes-no), New Onset (no-yes), Persistent (yes-yes).
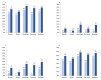
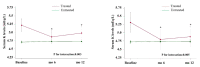
Results: We studied 562 patients (age 66.2 ± 14.5 y; 61% males; eGFR 39.8 ± 21.8 mL/min/1.73 m2, RAASI 76.2%). HK was "absent" in 50.7%, "resolving" in 15.6%, "new onset" in 16.6%, and "persistent" in 17.1%. Twenty-four hour urinary measurements testified adherence to nutritional recommendations in the four groups at either visit. We detected increased prescription from baseline to month 12 of bicarbonate supplements (from 5.0 to 14.1%, p < 0.0001), K-binders (from 2.0 to 7.7%, p < 0.0001), and non-K sparing diuretics (from 34.3 to 41.5%, p < 0.001); these changes were consistent across groups. Similar results were obtained when using higher sK level (≥5.5 mEq/L) to stratify patients. Mixed-effects regression analysis showed that higher sK over time was associated with eGFR < 60, diabetes, lower serum bicarbonate, lower use of non-K sparing diuretics, bicarbonate supplementation, and K-binder use. Treatment-by-time interaction showed that sK decreased in HK patients given bicarbonate (p = 0.003) and K-binders (p = 0.005).
Conclusions: This observational study discloses that one-third of ND-CKD patients under nephrology care remain with or develop HK during a 12-month period despite low K intake and increased use of sK-lowering drugs.
Keywords: CKD; RAASI; diet; hyperkalemia; potassium.
Conflict of interest statement
Roberto Minutolo has been member of Advisory Boards for Astellas, Amgen, and invited speaker at meetings supported by Amgen, Vifor Pharma. Luca De Nicola has received fees for scientific consultation and/or lectures by Astellas, AstraZeneca, Mundibiopharma and Vifor Pharma. Adamasco Cupisti has received consultation or speaker honoraria from Fresenius Kabi, Shaer, Vifor Pharma. Vincenzo Bellizzi has received fees for advisory boards and lectures from Shaer and Fresenius Kabi. Silvio Borrelli, Giuseppe Conte, Paolo Chiodini, Domenico Santoro, Vincenzo Calabrese, Domenico Giannese, Carlo Garofalo, Michele Provenzano, Luca Apicella, Giorgina Barbara Piccoli, Massimo Torreggiani, Biagio Di Iorio declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Kovesdy C.P., Matsushita K., Sang Y., Brunskill N.J., Carrero J.J., Chodick G., Hasegawa T., Heerspink H.L., Hirayama A., Landman G.W.D., et al. Serum potassium and adverse outcomes across the range of kidney function: A CKD Prognosis Consortium meta-analysis. Eur. Heart J. 2018;39:1535–1542. doi: 10.1093/eurheartj/ehy100. - DOI - PMC - PubMed
-
- Provenzano M., De Francesco M., Iannazzo S., Garofalo C., Andreucci M., Genualdo R., Borrelli S., Minutolo R., Conte G., De Nicola L. Cost-analysis of persistent hyperkalaemia in non-dialysis chronic kidney disease patients under nephrology care in Italy. Int. J. Clin. Pract. 2020;74:e13475. doi: 10.1111/ijcp.13475. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

