Phenolic Compounds and Biological Activity of Selected Mentha Species
- PMID: 33804017
- PMCID: PMC8000339
- DOI: 10.3390/plants10030550
Phenolic Compounds and Biological Activity of Selected Mentha Species
Abstract
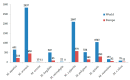
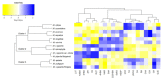
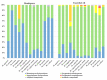
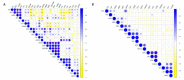
Mentha species are widely used as food, medicine, spices, and flavoring agents. Thus, chemical composition is an important parameter for assessing the quality of mints. In general, the contents of menthol, menthone, eucalyptol, and limonene comprise one of the major parameters for assessing the quality of commercially important mints. Building further on the phytochemical characterization of the quality of Mentha species, this work was focused on the composition of phenolic compounds in methanolic extracts. Thirteen Mentha species were grown under the same environmental conditions, and their methanolic extracts were subjected to the LC-MS/MS (liquid chromatography-tandem mass spectrometry) profiling of phenolics and the testing their biological activities, i.e., antioxidant and tyrosinase inhibition activities, which are important features for the cosmetic industry. The total phenolic content (TPC) ranged from 14.81 ± 1.09 mg GAE (gallic acid equivalents)/g for Mentha cervina to 58.93. ± 8.39 mg GAE/g for Mentha suaveolens. The antioxidant activity of examined Mentha related with the content of the phenolic compounds and ranged from 22.79 ± 1.85 to 106.04 ± 3.26 mg TE (Trolox equivalents)/g for M. cervina and Mentha x villosa, respectively. Additionally, Mentha pulegium (123.89 ± 5.64 mg KAE (kojic acid equivalents)/g) and Mentha x piperita (102.82 ± 15.16 mg KAE/g) showed a strong inhibition of the enzyme tyrosinase, which is related to skin hyperpigmentation. The most abundant compound in all samples was rosmarinic acid, ranging from 1363.38 ± 8323 to 2557.08 ± 64.21 μg/g. In general, the levels of phenolic acids in all examined mint extracts did not significantly differ. On the contrary, the levels of flavonoids varied within the species, especially in the case of hesperidin (from 0.73 ± 0.02 to 109. 39 ± 2.01 μg/g), luteolin (from 1.84 ± 0.11 to 31.03 ± 0.16 μg/g), and kaempferol (from 1.30 ± 0.17 to 33.68 ± 0.81 μg/g). Overall results indicated that all examined mints possess significant amounts of phenolic compounds that are responsible for antioxidant activity and, to some extent, for tyrosinase inhibition activity. Phenolics also proved to be adequate compounds, together with terpenoids, for the characterization of Mentha sp. Additionally, citrus-scented Mentha x villosa could be selected as a good candidate for the food and pharmaceutical industry, especially due its chemical composition and easy cultivation, even in winter continental conditions.
Keywords: LC–MS/MS; Mentha sp.; antioxidant activity; phenolic compounds; tyrosinase inhibition activity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Rice Evans C., Miler N., Paganga G. Antioxidant properties of phenolic compounds. Trend. Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. - DOI
-
- European Pharmacopoeia. 4th ed. Council of Europe; Strasbourg, France: 2002.
-
- Tucker A.O. Mentha: Economic uses. In: Lawrence B.M., editor. Mint: The Genus Mentha. CRC Press; Boca Raton, FL, USA: 2007. pp. 519–528.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

