Dinuclear Lanthanide(III) Complexes from the Use of Methyl 2-Pyridyl Ketoxime: Synthetic, Structural, and Physical Studies
- PMID: 33804026
- PMCID: PMC7999197
- DOI: 10.3390/molecules26061622
Dinuclear Lanthanide(III) Complexes from the Use of Methyl 2-Pyridyl Ketoxime: Synthetic, Structural, and Physical Studies
Abstract
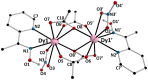
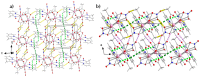
The first use of methyl 2-pyridyl ketoxime (mepaoH) in homometallic lanthanide(III) [Ln(III)] chemistry is described. The 1:2 reactions of Ln(NO3)3·nH2O (Ln = Nd, Eu, Gd, Tb, Dy; n = 5, 6) and mepaoH in MeCN have provided access to complexes [Ln2(O2CMe)4(NO3)2(mepaoH)2] (Ln = Nd, 1; Ln = Eu, 2; Ln = Gd, 3; Ln = Tb, 4; Ln = Dy, 5); the acetato ligands derive from the LnIII-mediated hydrolysis of MeCN. The 1:1 and 1:2 reactions between Dy(O2CMe)3·4H2O and mepaoH in MeOH/MeCN led to the all-acetato complex [Dy2(O2CMe)6(mepaoH)2] (6). Treatment of 6 with one equivalent of HNO3 gave 5. The structures of 1, 5, and 6 were solved by single-crystal X-ray crystallography. Elemental analyses and IR spectroscopy provide strong evidence that 2-4 display similar structural characteristics with 1 and 5. The structures of 1-5 consist of dinuclear molecules in which the two LnIII centers are bridged by two bidentate bridging (η1:η1:μ2) and two chelating-bridging (η1:η2:μ2) acetate groups. The LnIII atoms are each chelated by a N,N'-bidentate mepaoH ligand and a near-symmetrical bidentate nitrato group. The molecular structure of 6 is similar to that of 5, the main difference being the presence of two chelating acetato groups in the former instead of the two chelating nitrato groups in the latter. The geometry of the 9-coordinate LnIII centers in 1, 5 and 6 can be best described as a muffin-type (MFF-9). The 3D lattices of the isomorphous 1 and 5 are built through H-bonding, π⋯π stacking and C-H⋯π interactions, while the 3D architecture of 6 is stabilized by H bonds. The IR spectra of the complexes are discussed in terms of the coordination modes of the organic and inorganic ligands involved. The Eu(III) complex 2 displays a red, metal-ion centered emission in the solid state; the TbIII atom in solid 4 emits light in the same region with the ligand. Magnetic susceptibility studies in the 2.0-300 K range reveal weak antiferromagnetic intramolecular GdIII…GdIII exchange interactions in 3; the J value is -0.09(1) cm-1 based on the spin Hamiltonian Ĥ = -J(ŜGd1·ŜGd2).
Keywords: coordination chemistry; dinuclear lanthanide(III) complexes; magnetic properties of gadolinium(III) complexes; metal complexes of methyl 2-pyridyl ketoxime; photoluminescence studies; single-crystal X-ray structures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

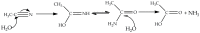






References
-
- Gerasimchuk N. Recent Advances in Chemistry and Applications of Oximes and their Metal Complexes: Parts I and II. Curr. Inorg. Chem. 2015;5:82. doi: 10.2174/187794410501150630184338. - DOI
-
- Tschugaeff L. Ueber ein neues, empfindliches reagens auf nickel. Ber. Dtsch. Chem. 1905;38:2520–2522. doi: 10.1002/cber.19050380317. - DOI
-
- Thorpe J.M., Beddoes R.L., Collison D., Garner C.D., Helliwell M., Holmes J.M., Tasker P.A. Surface coordination chemistry. Corrosion inhibition by tetranuclear cluster formation of iron with salicyladoxime. Angew. Chem. Int. Ed. 1999;38:1119–1121. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1119::AID-ANIE1119>3.0.CO;2-4. - DOI - PubMed
-
- Tasker P.A., Tong C.C., Westra A.N. Co-extraction of cations and anions in base metal recovery. Coord. Chem. Rev. 2007;251:1868–1877. doi: 10.1016/j.ccr.2007.03.014. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

