Hyalectanase Activities by the ADAMTS Metalloproteases
- PMID: 33804223
- PMCID: PMC8000579
- DOI: 10.3390/ijms22062988
Hyalectanase Activities by the ADAMTS Metalloproteases
Abstract
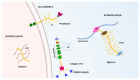
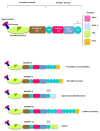
The hyalectan family is composed of the proteoglycans aggrecan, versican, brevican and neurocan. Hyalectans, also known as lecticans, are components of the extracellular matrix of different tissues and play essential roles in key biological processes including skeletal development, and they are related to the correct maintenance of the vascular and central nervous system. For instance, hyalectans participate in the organization of structures such as perineural nets and in the regulation of neurite outgrowth or brain recovery following a traumatic injury. The ADAMTS (A Disintegrin and Metalloprotease domains, with thrombospondin motifs) family consists of 19 secreted metalloproteases. These enzymes also perform important roles in the structural organization and function of the extracellular matrix through interactions with other matrix components or as a consequence of their catalytic activity. In this regard, some of their preferred substrates are the hyalectans. In fact, ADAMTSs cleave hyalectans not only as a mechanism for clearance or turnover of proteoglycans but also to generate bioactive fragments which display specific functions. In this article we review some of the physiological and pathological effects derived from cleavages of hyalectans mediated by ADAMTSs.
Keywords: ADAMTS; aggrecan; brevican; extracellular matrix; hyalectan; lectican; neurocan; proteoglycan; versican.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

