Quantitative Assessment of Occipital Metabolic and Energetic Changes in Parkinson's Patients, Using In Vivo 31P MRS-Based Metabolic Imaging at 7T
- PMID: 33804401
- PMCID: PMC8000945
- DOI: 10.3390/metabo11030145
Quantitative Assessment of Occipital Metabolic and Energetic Changes in Parkinson's Patients, Using In Vivo 31P MRS-Based Metabolic Imaging at 7T
Abstract
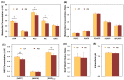
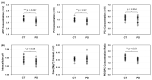
Abnormal energy metabolism associated with mitochondrial dysfunction is thought to be a major contributor to the progression of neurodegenerative diseases such as Parkinson's disease (PD). Recent advancements in the field of magnetic resonance (MR) based metabolic imaging provide state-of-the-art technologies for non-invasively probing cerebral energy metabolism under various brain conditions. In this proof-of-principle clinical study, we employed quantitative 31P MR spectroscopy (MRS) imaging techniques to determine a constellation of metabolic and bioenergetic parameters, including cerebral adenosine triphosphate (ATP) and other phosphorous metabolite concentrations, intracellular pH and nicotinamide adenine dinucleotide (NAD) redox ratio, and ATP production rates in the occipital lobe of cognitive-normal PD patients, and then we compared them with age-sex matched healthy controls. Small but statistically significant differences in intracellular pH, NAD and ATP contents and ATPase enzyme activity between the two groups were detected, suggesting that subtle defects in energy metabolism and mitochondrial function are quantifiable before regional neurological deficits or pathogenesis begin to occur in these patients. Pilot data aiming to evaluate the bioenergetic effect of mitochondrial-protective bile acid, ursodeoxycholic acid (UDCA) were also obtained. These results collectively demonstrated that in vivo 31P MRS-based neuroimaging can non-invasively and quantitatively assess key metabolic-energetic metrics in the human brain. This provides an exciting opportunity to better understand neurodegenerative diseases, their progression and response to treatment.
Keywords: cerebral ATP energy metabolism; human brain; in vivo 31P MRS-based metabolic imaging; neurodegenerative disease; ursodeoxycholic acid (UDCA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Siesjo B.K. Brain Energy Metabolism. Wiley; Hoboken, NJ, USA: 1978.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

