Differential Morpho-Physiological and Transcriptomic Responses to Heat Stress in Two Blueberry Species
- PMID: 33804571
- PMCID: PMC7957502
- DOI: 10.3390/ijms22052481
Differential Morpho-Physiological and Transcriptomic Responses to Heat Stress in Two Blueberry Species
Abstract
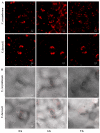
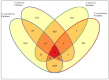
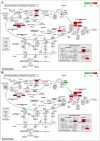
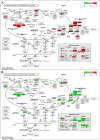
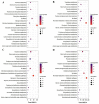
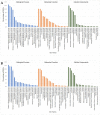
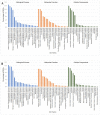
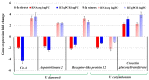
Blueberries (Vaccinium spp.) are highly vulnerable to changing climatic conditions, especially increasing temperatures. To gain insight into mechanisms underpinning the response to heat stress, two blueberry species were subjected to heat stress for 6 and 9 h at 45 °C, and leaf samples were used to study the morpho-physiological and transcriptomic changes. As compared with Vaccinium corymbosum, Vaccinium darrowii exhibited thermal stress adaptation features such as small leaf size, parallel leaf orientation, waxy leaf coating, increased stomatal surface area, and stomatal closure. RNAseq analysis yielded ~135 million reads and identified 8305 differentially expressed genes (DEGs) during heat stress against the control samples. In V. corymbosum, 2861 and 4565 genes were differentially expressed at 6 and 9 h of heat stress, whereas in V. darrowii, 2516 and 3072 DEGs were differentially expressed at 6 and 9 h, respectively. Among the pathways, the protein processing in the endoplasmic reticulum (ER) was the highly enriched pathway in both the species: however, certain metabolic, fatty acid, photosynthesis-related, peroxisomal, and circadian rhythm pathways were enriched differently among the species. KEGG enrichment analysis of the DEGs revealed important biosynthesis and metabolic pathways crucial in response to heat stress. The GO terms enriched in both the species under heat stress were similar, but more DEGs were enriched for GO terms in V. darrowii than the V. corymbosum. Together, these results elucidate the differential response of morpho-physiological and molecular mechanisms used by both the blueberry species under heat stress, and help in understanding the complex mechanisms involved in heat stress tolerance.
Keywords: RNAseq; blueberry; differentially expressed genes; heat stress; pathway analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Transcriptomic Analysis of Heat Stress Response in Brassica rapa L. ssp. pekinensis with Improved Thermotolerance through Exogenous Glycine Betaine.Int J Mol Sci. 2023 Mar 29;24(7):6429. doi: 10.3390/ijms24076429. Int J Mol Sci. 2023. PMID: 37047402 Free PMC article.
-
Regulation of gene expression in roots of the pH-sensitive Vaccinium corymbosum and the pH-tolerant Vaccinium arboreum in response to near neutral pH stress using RNA-Seq.BMC Genomics. 2017 Aug 7;18(1):580. doi: 10.1186/s12864-017-3967-0. BMC Genomics. 2017. PMID: 28784085 Free PMC article.
-
Chromosome-level genome assembly of the diploid blueberry Vaccinium darrowii provides insights into its subtropical adaptation and cuticle synthesis.Plant Commun. 2022 Jul 11;3(4):100307. doi: 10.1016/j.xplc.2022.100307. Epub 2022 Feb 26. Plant Commun. 2022. PMID: 35605198 Free PMC article.
-
Transcriptome analysis of postharvest blueberries (Vaccinium corymbosum 'Duke') in response to cold stress.BMC Plant Biol. 2020 Feb 19;20(1):80. doi: 10.1186/s12870-020-2281-1. BMC Plant Biol. 2020. PMID: 32075582 Free PMC article.
-
Progress in Research on the Mechanisms Underlying Chloroplast-Involved Heat Tolerance in Plants.Genes (Basel). 2021 Aug 28;12(9):1343. doi: 10.3390/genes12091343. Genes (Basel). 2021. PMID: 34573325 Free PMC article. Review.
Cited by
-
Transcriptome Profiling of Two Camellia japonica Cultivars with Different Heat Tolerance Reveals Heat Stress Response Mechanisms.Plants (Basel). 2024 Nov 2;13(21):3089. doi: 10.3390/plants13213089. Plants (Basel). 2024. PMID: 39520009 Free PMC article.
-
Comparative Physiological and Transcriptomic Analyses of Improved Heat Stress Tolerance in Celery (Apium Graveolens L.) Caused by Exogenous Melatonin.Int J Mol Sci. 2022 Sep 27;23(19):11382. doi: 10.3390/ijms231911382. Int J Mol Sci. 2022. PMID: 36232683 Free PMC article.
-
Generation and characterization of novel transcriptome-derived SSR markers for genetic applications in blueberry.Sci Rep. 2025 Jul 11;15(1):25050. doi: 10.1038/s41598-025-10317-2. Sci Rep. 2025. PMID: 40646147 Free PMC article.
-
Comparative physiological and transcriptomic analyses identify computationally predicted key genes and regulatory pathways in non-heading Chinese cabbage under heat stress.BMC Plant Biol. 2025 Aug 8;25(1):1042. doi: 10.1186/s12870-025-07120-6. BMC Plant Biol. 2025. PMID: 40781588 Free PMC article.
-
Transcriptome profiling, physiological, and biochemical analyses provide new insights towards drought stress response in sugar maple (Acer saccharum Marshall) saplings.Front Plant Sci. 2023 Apr 19;14:1150204. doi: 10.3389/fpls.2023.1150204. eCollection 2023. Front Plant Sci. 2023. PMID: 37152134 Free PMC article.
References
-
- Retamales J.B., Hancock J.F. Blueberries. Volume 27 Cabi; Cambridge, MA, USA: 2018.
-
- Agricultural Marketing Resource Center Blueberries. [(accessed on 5 March 2020)]; Available online: https://www.agmrc.org/commodities-products/fruits/blueberries.
-
- Shi M., Loftus H., McAinch A.J., Su X.Q. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J. Funct. Foods. 2017;30:16–29. doi: 10.1016/j.jff.2016.12.036. - DOI
-
- Davies K., Espley R. Opportunities and challenges for metabolic engineering of secondary metabolite pathways for improved human health characters in fruit and vegetable crops. N. Z. J. Crop Hortic. Sci. 2013;41:154–177. doi: 10.1080/01140671.2013.793730. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

