Silica Monolith for the Removal of Pollutants from Gas and Aqueous Phases
- PMID: 33804572
- PMCID: PMC7957575
- DOI: 10.3390/molecules26051316
Silica Monolith for the Removal of Pollutants from Gas and Aqueous Phases
Abstract
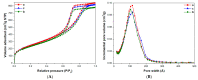
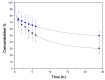
This study focused on the application of mesoporous silica monoliths for the removal of organic pollutants. The physico-chemical textural and surface properties of the monoliths were investigated. The homogeneity of the textural properties along the entire length of the monoliths was assessed, as well as the reproducibility of the synthesis method. The adsorption properties of the monoliths for gaseous toluene, as a model of Volatile Organic Compounds (VOCs), were evaluated and compared to those of a reference meso-structured silica powder (MCM-41) of commercial origin. Silica monoliths adsorbed comparable amounts of toluene with respect to MCM-41, with better performances at low pressure. Finally, considering their potential application in water phase, the adsorption properties of monoliths toward Rhodamine B, selected as a model molecule of water soluble pollutants, were studied together with their stability in water. After 24 h of contact, the silica monoliths were able to adsorb up to the 70% of 1.5 × 10-2 mM Rhodamine B in water solution.
Keywords: adsorption; rhodamine B; toluene; water stability of monolith.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Mesoporous aluminosilica monoliths for the adsorptive removal of small organic pollutants.J Hazard Mater. 2012 Jan 30;201-202:23-32. doi: 10.1016/j.jhazmat.2011.10.088. Epub 2011 Nov 7. J Hazard Mater. 2012. PMID: 22169140
-
Optimization of the single-step synthesis of hybrid C(8) silica monoliths dedicated to nano-liquid chromatography and capillary electrochromatography.J Chromatogr A. 2008 Oct 31;1209(1-2):120-7. doi: 10.1016/j.chroma.2008.08.119. Epub 2008 Sep 10. J Chromatogr A. 2008. PMID: 18814877
-
Adsorption and degradation of model volatile organic compounds by a combined titania-montmorillonite-silica photocatalyst.J Hazard Mater. 2011 Jun 15;190(1-3):416-23. doi: 10.1016/j.jhazmat.2011.03.064. Epub 2011 Mar 27. J Hazard Mater. 2011. PMID: 21501924
-
High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review.Water Res. 2018 Nov 1;144:145-161. doi: 10.1016/j.watres.2018.07.017. Epub 2018 Jul 7. Water Res. 2018. PMID: 30025266 Review.
-
Strategies based on silica monoliths for removing pollutants from wastewater effluents: a review.Sci Total Environ. 2013 Sep 1;461-462:126-38. doi: 10.1016/j.scitotenv.2013.04.091. Epub 2013 May 25. Sci Total Environ. 2013. PMID: 23714248 Review.
Cited by
-
CO2 Capture: A Comprehensive Review and Bibliometric Analysis of Scalable Materials and Sustainable Solutions.Molecules. 2025 Jan 26;30(3):563. doi: 10.3390/molecules30030563. Molecules. 2025. PMID: 39942667 Free PMC article. Review.
-
Nanosized MCM-41 silica from rice husk and its application for the removal of organic dyes from water.RSC Adv. 2025 Jan 24;15(4):2545-2553. doi: 10.1039/d4ra07152b. eCollection 2025 Jan 23. RSC Adv. 2025. PMID: 39867322 Free PMC article.
-
Water Decontamination from Cr(VI) by Transparent Silica Xerogel Monolith.Int J Mol Sci. 2023 Apr 18;24(8):7430. doi: 10.3390/ijms24087430. Int J Mol Sci. 2023. PMID: 37108592 Free PMC article.
References
-
- Fotoohi B., Kazemzad M., Mercier L. Additive-Free Synthesis of Robust Monolithic Mesoporous Silica Support Used in Catalysis. Ceram. Int. 2018;44:20199–20210. doi: 10.1016/j.ceramint.2018.08.003. - DOI
-
- Michels N.-L., Mitchell S., Pérez-Ramírez J. Effects of Binders on the Performance of Shaped Hierarchical MFI Zeolites in Methanol-to-Hydrocarbons. ACS Catal. 2014;4:2409–2417. doi: 10.1021/cs500353b. - DOI
-
- Tomašić V., Jović F. State-of-the-Art in the Monolithic Catalysts/Reactors. Appl. Catal. A: Gen. 2006;311:112–121. doi: 10.1016/j.apcata.2006.06.013. - DOI
-
- Galarneau A., Abid Z., Said B., Didi Y., Szymanska K., Jarzębski A., Tancret F., Hamaizi H., Bengueddach A., Di Renzo F., et al. Synthesis and Textural Characterization of Mesoporous and Meso-/Macroporous Silica Monoliths Obtained by Spinodal Decomposition. Inorganics. 2016;4:9. doi: 10.3390/inorganics4020009. - DOI
-
- Amatani T., Nakanishi K., Hirao K., Kodaira T. Monolithic Periodic Mesoporous Silica with Well-Defined Macropores. Chem. Mater. 2005;17:2114–2119. doi: 10.1021/cm048091c. - DOI
MeSH terms
Substances
Grants and funding
- 2° Bando "Proof of Concept d'Ateneo" (PoC UNIBO) - Finanziamento a supporto dello sviluppo di invenzioni brevettate"/2° Bando "Proof of Concept d'Ateneo" (PoC UNIBO) - Finanziamento a supporto dello sviluppo di invenzioni brevettate"
- "Bando per la realizzazione di programmi di valorizzazione dei brevetti tramite il finanziamento di progetti di Proof of Concept (PoC) delle Università italiane, degli Enti Pubblici di Ricerca (EPR) italiani e degli Istituti di ricovero e cura a carattere/"Bando per la realizzazione di programmi di valorizzazione dei brevetti tramite il finanziamento di progetti di Proof of Concept (PoC) delle Università italiane, degli Enti Pubblici di Ricerca (EPR) italiani e degli Istituti di ricovero e cura a carattere
LinkOut - more resources
Full Text Sources
Other Literature Sources

