Development of a New Highly Selective Monoclonal Antibody against Preferentially Expressed Antigen in Melanoma (PRAME) and Identification of the Target Epitope by Bio-Layer Interferometry
- PMID: 33804612
- PMCID: PMC8003813
- DOI: 10.3390/ijms22063166
Development of a New Highly Selective Monoclonal Antibody against Preferentially Expressed Antigen in Melanoma (PRAME) and Identification of the Target Epitope by Bio-Layer Interferometry
Abstract
Background: Monoclonal antibodies (mAbs) against cancer biomarkers are key reagents in diagnosis and therapy. One such relevant biomarker is a preferentially expressed antigen in melanoma (PRAME) that is selectively expressed in many tumors. Knowing mAb's epitope is of utmost importance for understanding the potential activity and therapeutic prospective of the reagents.
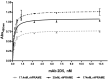
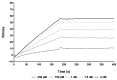
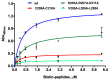
Methods: We generated a mAb against PRAME immunizing mice with PRAME fragment 161-415; the affinity of the antibody for the protein was evaluated by ELISA and SPR, and its ability to detect the protein in cells was probed by cytofluorimetry and Western blotting experiments. The antibody epitope was identified immobilizing the mAb on bio-layer interferometry (BLI) sensor chip, capturing protein fragments obtained following trypsin digestion and performing mass spectrometry analyses.
Results: A mAb against PRAME with an affinity of 35 pM was obtained and characterized. Its epitope on PRAME was localized on residues 202-212, taking advantage of the low volumes and lack of fluidics underlying the BLI settings.
Conclusions: The new anti-PRAME mAb recognizes the folded protein on the surface of cell membranes suggesting that the antibody's epitope is well exposed. BLI sensor chips can be used to identify antibody epitopes.
Keywords: PRAME; bio-layer interferometry; epitope identification; mAb.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ikeda H., Lethe B., Lehmann F., van Baren N., Baurain J.F., de Smet C., Chambost H., Vitale M., Moretta A., Boon T., et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208. doi: 10.1016/S1074-7613(00)80426-4. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- Research Project on CAR-T cells for haematological malignancies and solid tumors/Ministero della Salute
- Development of novel therapeutic approaches for treatment resistant neoplastic diseases (SATIN)/Regione Campania
- Fighting Cancer resistance: Multidisciplinary integrated Platform for a technological Innovative Approach to Oncotherapies (Campania Oncotherapies)/Regione Campania
- NANOCAN, NANOfotonica per la lotta al CANcro/Regione Campania
LinkOut - more resources
Full Text Sources
Other Literature Sources

