Noncovalent Bonds through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons
- PMID: 33804617
- PMCID: PMC8003638
- DOI: 10.3390/molecules26061740
Noncovalent Bonds through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons
Abstract
Over the last years, scientific interest in noncovalent interactions based on the presence of electron-depleted regions called σ-holes or π-holes has markedly accelerated. Their high directionality and strength, comparable to hydrogen bonds, has been documented in many fields of modern chemistry. The current review gathers and digests recent results concerning these bonds, with a focus on those systems where both σ and π-holes are present on the same molecule. The underlying principles guiding the bonding in both sorts of interactions are discussed, and the trends that emerge from recent work offer a guide as to how one might design systems that allow multiple noncovalent bonds to occur simultaneously, or that prefer one bond type over another.
Keywords: chalcogen bond; cooperativity; halogen bond; molecular electrostatic potential; pnicogen bond; tetrel bond.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



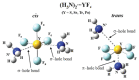
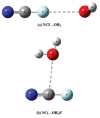
References
-
- Guthrie F. On the Iodide of Iodammonium. J. Chem. Soc. 1863;16:239–244. doi: 10.1039/JS8631600239. - DOI
-
- Remsen I., Norris J.F. Action of the halogens on the methyl-amines. Am. Chem. J. 1896;18:90–96.
-
- Hope H., McCullough J.D. The crystal structure of the molecular complex of iodine with tetrahydroselenophene, C4H8Se.I2. Acta Cryst. 1964;17:712–718. doi: 10.1107/S0365110X64001773. - DOI
-
- Olie K., Mijlhoff F.C. The crystal structure of POBr3 and intermolecular bonding. Acta Cryst. Sect. B. 1969;25:974–977. doi: 10.1107/S056774086900327X. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

