P-Glycoprotein Inhibitor Tariquidar Plays an Important Regulatory Role in Pigmentation in Larval Zebrafish
- PMID: 33804686
- PMCID: PMC8003715
- DOI: 10.3390/cells10030690
P-Glycoprotein Inhibitor Tariquidar Plays an Important Regulatory Role in Pigmentation in Larval Zebrafish
Abstract
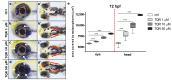
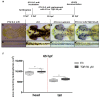
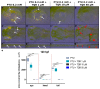
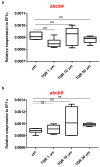
Zebrafish has emerged as a powerful model in studies dealing with pigment development and pathobiology of pigment diseases. Due to its conserved pigment pattern with established genetic background, the zebrafish is used for screening of active compounds influencing melanophore, iridophore, and xanthophore development and differentiation. In our study, zebrafish embryos and larvae were used to investigate the influence of third-generation noncompetitive P-glycoprotein inhibitor, tariquidar (TQR), on pigmentation, including phenotype effects and changes in gene expression of chosen chromatophore differentiation markers. Five-day exposure to increasing TQR concentrations (1 µM, 10 µM, and 50 µM) resulted in a dose-dependent augmentation of the area covered with melanophores but a reduction in the area covered by iridophores. The observations were performed in three distinct regions-the eye, dorsal head, and tail. Moreover, TQR enhanced melanophore renewal after depigmentation caused by 0.2 mM 1-phenyl-2-thiourea (PTU) treatment. qPCR analysis performed in 56-h post-fertilization (hpf) embryos demonstrated differential expression patterns of genes related to pigment development and differentiation. The most substantial findings include those indicating that TQR had no significant influence on leukocyte tyrosine kinase, GTP cyclohydrolase 2, tyrosinase-related protein 1, and forkhead box D3, however, markedly upregulated tyrosinase, dopachrome tautomerase and melanocyte inducing transcription factor, and downregulated purine nucleoside phosphorylase 4a. The present study suggests that TQR is an agent with multidirectional properties toward pigment cell formation and distribution in the zebrafish larvae and therefore points to the involvement of P-glycoprotein in this process.
Keywords: P-glycoprotein inhibitor; dopachrome tautomerase; iridophores; melanocyte inducing transcription factor; melanophores; pigment cells; purine nucleoside phosphorylase 4a; tariquidar; tyrosinase; zebrafish.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

