Voluntary Wheel Running Did Not Alter Gene Expression in 5xfad Mice, but in Wild-Type Animals Exclusively after One-Day of Physical Activity
- PMID: 33804749
- PMCID: PMC8004053
- DOI: 10.3390/cells10030693
Voluntary Wheel Running Did Not Alter Gene Expression in 5xfad Mice, but in Wild-Type Animals Exclusively after One-Day of Physical Activity
Abstract
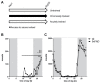
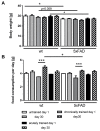
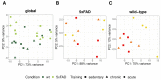
Physical activity is considered a promising preventive intervention to reduce the risk of developing Alzheimer's disease (AD). However, the positive effect of therapeutic administration of physical activity has not been proven conclusively yet, likely due to confounding factors such as varying activity regimens and life or disease stages. To examine the impact of different routines of physical activity in the early disease stages, we subjected young 5xFAD and wild-type mice to 1-day (acute) and 30-day (chronic) voluntary wheel running and compared them with age-matched sedentary controls. We observed a significant increase in brain lactate levels in acutely trained 5xFAD mice relative to all other experimental groups. Subsequent brain RNA-seq analysis did not reveal major differences in transcriptomic regulation between training durations in 5xFAD mice. In contrast, acute training yielded substantial gene expression changes in wild-type animals relative to their chronically trained and sedentary counterparts. The comparison of 5xFAD and wild-type mice showed the highest transcriptional differences in the chronic and sedentary groups, whereas acute training was associated with much fewer differentially expressed genes. In conclusion, our results suggest that different training durations did not affect the global transcriptome of 3-month-old 5xFAD mice, whereas acute running seemed to induce a similar transcriptional stress state in wild-type animals as already known for 5xFAD mice.
Keywords: 5xFAD; Alzheimer’s disease; acute; chronic; physical activity; wheel running.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. - DOI - PMC - PubMed
-
- Conde-Sala J.L., Turró-Garriga O., Piñán-Hernández S., Portellano-Ortiz C., Viñas-Diez V., Gascón-Bayarri J., Reñé-Ramírez R. Effects of Anosognosia and Neuropsychiatric Symptoms on the Quality of Life of Patients with Alzheimer’s Disease: A 24-Month Follow-up Study. Int. J. Geriatr. Psychiatry. 2016;31:109–119. doi: 10.1002/gps.4298. - DOI - PubMed
-
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

