The Function of Sialidase Revealed by Sialidase Activity Imaging Probe
- PMID: 33804798
- PMCID: PMC8003999
- DOI: 10.3390/ijms22063187
The Function of Sialidase Revealed by Sialidase Activity Imaging Probe
Abstract
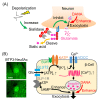
Sialidase cleaves sialic acid residues from glycans such as glycoproteins and glycolipids. In the brain, desorption of the sialic acid by sialidase is essential for synaptic plasticity, learning and memory and synaptic transmission. BTP3-Neu5Ac has been developed for sensitive imaging of sialidase enzyme activity in mammalian tissues. Sialidase activity in the rat hippocampus detected with BTP3-Neu5Ac increases rapidly by neuronal depolarization. It is presumed that an increased sialidase activity in conjunction with neural excitation is involved in the formation of the neural circuit for memory. Since sialidase inhibits the exocytosis of the excitatory neurotransmitter glutamate, the increased sialidase activity by neural excitation might play a role in the negative feedback mechanism against the glutamate release. Mammalian tissues other than the brain have also been stained with BTP3-Neu5Ac. On the basis of information on the sialidase activity imaging in the pancreas, it was found that sialidase inhibitor can be used as an anti-diabetic drug that can avoid hypoglycemia, a serious side effect of insulin secretagogues. In this review, we discuss the role of sialidase in the brain as well as in the pancreas and skin, as revealed by using a sialidase activity imaging probe. We also present the detection of influenza virus with BTP3-Neu5Ac and modification of BTP3-Neu5Ac.
Keywords: BTP3-Neu5Ac; BTP9-Neu5Ac; diabetes; elastin; glutamate; hippocampus; memory; pancreas; sialidase; skin; virus.
Conflict of interest statement
The authors declare that they have no conflict of interest with the contents of this article.
Figures


Similar articles
-
A novel method for detection of Newcastle disease virus with a fluorescent sialidase substrate.J Virol Methods. 2014 Dec;209:136-42. doi: 10.1016/j.jviromet.2014.09.010. Epub 2014 Sep 20. J Virol Methods. 2014. PMID: 25241143
-
Rapid regulation of sialidase activity in response to neural activity and sialic acid removal during memory processing in rat hippocampus.J Biol Chem. 2017 Apr 7;292(14):5645-5654. doi: 10.1074/jbc.M116.764357. Epub 2017 Feb 17. J Biol Chem. 2017. PMID: 28213516 Free PMC article.
-
Fluorogenic Probes for Accurate in Situ Imaging of Viral and Mammalian Sialidases.ACS Chem Biol. 2019 Jun 21;14(6):1195-1204. doi: 10.1021/acschembio.9b00103. Epub 2019 Jun 10. ACS Chem Biol. 2019. PMID: 31120724
-
High efficiency method of detection and isolation of neuraminidase inhibitor resistant influenza viruses by fluorescence sialidase imaging.Jpn J Antibiot. 2016 Dec;69(6):357-366. Jpn J Antibiot. 2016. PMID: 30226930 Review. English, Japanese.
-
Imaging of Sialidase Activity and Its Clinical Application.Biol Pharm Bull. 2017;40(12):2015-2023. doi: 10.1248/bpb.b17-00592. Biol Pharm Bull. 2017. PMID: 29199226 Review.
Cited by
-
Safe Sialidase Production by the Saprophyte Oerskovia paurometabola: Gene Sequence and Enzyme Purification.Molecules. 2022 Dec 15;27(24):8922. doi: 10.3390/molecules27248922. Molecules. 2022. PMID: 36558051 Free PMC article.
-
Sialic acid as the potential link between lipid metabolism and inflammation in the pathogenesis of atherosclerosis.Braz J Med Biol Res. 2023 Dec 11;56:e12972. doi: 10.1590/1414-431X2023e12972. eCollection 2023. Braz J Med Biol Res. 2023. PMID: 38088673 Free PMC article. Review.
-
Polysialylation of Glioblastoma Cells Is Regulated by Autophagy Under Nutrient Deprivation.Int J Mol Sci. 2025 Aug 6;26(15):7625. doi: 10.3390/ijms26157625. Int J Mol Sci. 2025. PMID: 40806752 Free PMC article.
-
Autologous blood in the management of ocular surface disorders.World J Exp Med. 2024 Dec 20;14(4):96412. doi: 10.5493/wjem.v14.i4.96412. eCollection 2024 Dec 20. World J Exp Med. 2024. PMID: 39713083 Free PMC article. Review.
References
-
- Hammond M.S., Sims C., Parameshwaran K., Suppiramaniam V., Schachner M., Dityatev A. Neural cell adhesion molecule-associated polysialic acid inhibits NR2B-containing N-methyl-D-aspartate receptors and prevents glutamate-induced cell death. J. Biol. Chem. 2006;281:34859–34869. doi: 10.1074/jbc.M602568200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

