Systemic Therapy of Metastatic Melanoma: On the Road to Cure
- PMID: 33804800
- PMCID: PMC8003858
- DOI: 10.3390/cancers13061430
Systemic Therapy of Metastatic Melanoma: On the Road to Cure
Abstract
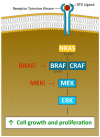
This decade has brought significant survival improvement in patients with metastatic melanoma with targeted therapies and immunotherapies. As our understanding of the mechanisms of action of these therapeutics evolves, even more impressive therapeutic success is being achieved through various combination strategies, including combinations of different immunotherapies as well as with other modalities. This review summarizes prospectively and retrospectively generated clinical evidence on modern melanoma therapy, focusing on immunotherapy and targeted therapy with BRAF kinase inhibitors and MEK kinase inhibitors (BRAF/MEK inhibitors), including recent data presented at major conference meetings. The combination of the anti-PD-1 directed monoclonal antibody nivolumab and of the CTLA-4 antagonist ipilimumab achieves unprecedented 5-year overall survival (OS) rates above 50%; however, toxicity is high. For PD-1 monotherapy (nivolumab or pembrolizumab), toxicities are in general well manageable. Today, novel combinations of such immune checkpoint inhibitors (ICIs) are under investigation, for example with cytokines and oncolytic viruses (i.e., pegylated interleukin-2, talimogene laherparepvec). Furthermore, current studies investigate the combined or sequential use of ICIs plus BRAF/MEK inhibitors. Several studies focus particularly on poor prognosis patients, as e.g., on anti-PD-1 refractory melanoma, patients with brain metastases, or uveal melanoma. It is hoped, on the road to cure, that these new approaches further improve long term survival in patients with advanced or metastatic melanoma.
Keywords: BRAF inhibitors; Immune checkpoint inhibitors; MEK inhibitors; melanoma; systemic therapy.
Conflict of interest statement
Friedegund Meier has received travel support or/and speaker fees or/and consultant fees from Novartis, Roche, BMS, MSD and Pierre Fabre as well as research funding from Novartis and Roche. The other authors have no conflicts of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

