Osmolality Effects on CHO Cell Growth, Cell Volume, Antibody Productivity and Glycosylation
- PMID: 33804825
- PMCID: PMC8037477
- DOI: 10.3390/ijms22073290
Osmolality Effects on CHO Cell Growth, Cell Volume, Antibody Productivity and Glycosylation
Abstract
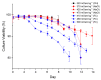
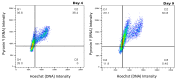
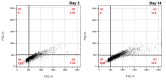
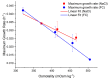
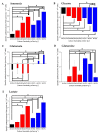
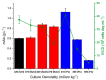
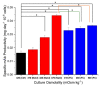
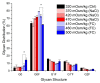
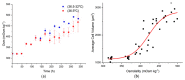
The addition of nutrients and accumulation of metabolites in a fed-batch culture of Chinese hamster ovary (CHO) cells leads to an increase in extracellular osmolality in late stage culture. Herein, we explore the effect of osmolality on CHO cell growth, specific monoclonal antibody (mAb) productivity and glycosylation achieved with the addition of NaCl or the supplementation of a commercial feed. Although both methods lead to an increase in specific antibody productivity, they have different effects on cell growth and antibody production. Osmolality modulation using NaCl up to 470 mOsm kg-1 had a consistently positive effect on specific antibody productivity and titre. The addition of the commercial feed achieved variable results: specific mAb productivity was increased, yet cell growth rate was significantly compromised at high osmolality values. As a result, Feed C addition to 410 mOsm kg-1 was the only condition that achieved a significantly higher mAb titre compared to the control. Additionally, Feed C supplementation resulted in a significant reduction in galactosylated antibody structures. Cell volume was found to be positively correlated to osmolality; however, osmolality alone could not account for observed changes in average cell diameter without considering cell cycle variations. These results help delineate the overall effect of osmolality on titre and highlight the potentially negative effect of overfeeding on cell growth.
Keywords: Chinese hamster ovary cells; antibody glycosylation; cell volume; hyperosmolality; monoclonal antibody.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Hendrick V., Winnepenninckx P., Abdelkafi C., Vandeputte O., Cherlet M., Marique T., Renemann G., Loa A., Kretzmer G., Werenne J. Increased productivity of re-combinant tissular plasminogen activator (t-PA) by butyrate and shift of temperature: A cell cycle phases analysis. Cytotechnology. 2001;36:71–83. doi: 10.1023/A:1014088919546. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

