Circulation of Babesia Species and Their Exposure to Humans through Ixodes Ricinus
- PMID: 33804875
- PMCID: PMC8063829
- DOI: 10.3390/pathogens10040386
Circulation of Babesia Species and Their Exposure to Humans through Ixodes Ricinus
Abstract
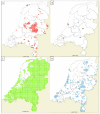
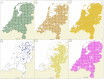
Human babesiosis in Europe has been attributed to infection with Babesia divergens and, to a lesser extent, with Babesia venatorum and Babesia microti, which are all transmitted to humans through a bite of Ixodes ricinus. These Babesia species circulate in the Netherlands, but autochthonous human babesiosis cases have not been reported so far. To gain more insight into the natural sources of these Babesia species, their presence in reservoir hosts and in I. ricinus was examined. Moreover, part of the ticks were tested for co-infections with other tick borne pathogens. In a cross-sectional study, qPCR-detection was used to determine the presence of Babesia species in 4611 tissue samples from 27 mammalian species and 13 bird species. Reverse line blotting (RLB) and qPCR detection of Babesia species were used to test 25,849 questing I. ricinus. Fragments of the 18S rDNA and cytochrome c oxidase subunit I (COI) gene from PCR-positive isolates were sequenced for confirmation and species identification and species-specific PCR reactions were performed on samples with suspected mixed infections. Babesia microti was found in two widespread rodent species: Myodes glareolus and Apodemus sylvaticus, whereas B. divergens was detected in the geographically restricted Cervus elaphus and Bison bonasus, and occasionally in free-ranging Ovis aries. B. venatorum was detected in the ubiquitous Capreolus capreolus, and occasionally in free-ranging O. aries. Species-specific PCR revealed co-infections in C. capreolus and C. elaphus, resulting in higher prevalence of B. venatorum and B. divergens than disclosed by qPCR detection, followed by 18S rDNA and COI sequencing. The non-zoonotic Babesia species found were Babesia capreoli, Babesia vulpes, Babesia sp. deer clade, and badger-associated Babesia species. The infection rate of zoonotic Babesia species in questing I. ricinus ticks was higher for Babesia clade I (2.6%) than Babesia clade X (1.9%). Co-infection of B. microti with Borrelia burgdorferi sensu lato and Neoehrlichia mikurensis in questing nymphs occurred more than expected, which reflects their mutual reservoir hosts, and suggests the possibility of co-transmission of these three pathogens to humans during a tick bite. The ubiquitous spread and abundance of B. microti and B. venatorum in their reservoir hosts and questing ticks imply some level of human exposure through tick bites. The restricted distribution of the wild reservoir hosts for B. divergens and its low infection rate in ticks might contribute to the absence of reported autochthonous cases of human babesiosis in the Netherlands.
Keywords: Ixodes ricinus; One Health; babesiosis; disease risk; sylvatic cycle; zoonoses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

