In Vitro Study of the Fibrinolytic Activity via Single Chain Urokinase-Type Plasminogen Activator and Molecular Docking of FGFC1
- PMID: 33804930
- PMCID: PMC8036777
- DOI: 10.3390/molecules26071816
In Vitro Study of the Fibrinolytic Activity via Single Chain Urokinase-Type Plasminogen Activator and Molecular Docking of FGFC1
Abstract
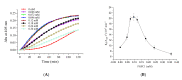
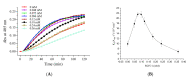
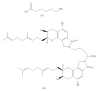
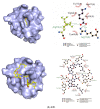
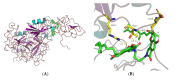
Fungi fibrinolytic compound 1 (FGFC1) is a rare marine-derived compound that can enhance fibrinolysis both in vitro and in vivo. The fibrinolytic activity characterization of FGFC1 mediated by plasminogen (Glu-/Lys-) and a single-chain urokinase-type plasminogen activator (pro-uPA) was further evaluated. The binding sites and mode of binding between FGFC1 and plasminogen were investigated by means of a combination of in vitro experiments and molecular docking. A 2.2-fold enhancement of fibrinolytic activity was achieved at 0.096 mM FGFC1, whereas the inhibition of fibrinolytic activity occurred when the FGFC1 concentration was above 0.24 mM. The inhibition of fibrinolytic activity of FGFC1 by 6-aminohexanoic acid (EACA) and tranexamic acid (TXA) together with the docking results revealed that the lysine-binding sites (LBSs) play a crucial role in the process of FGFC1 binding to plasminogen. The action mechanism of FGFC1 binding to plasminogen was inferred, and FGFC1 was able to induce plasminogen to exhibit an open conformation by binding through the LBSs. The molecular docking results showed that docking of ligands (EACA, FGFC1) with receptors (KR1-KR5) mainly occurred through hydrophilic and hydrophobic interactions. In addition, the binding affinity values of EACA to KR1-KR5 were -5.2, -4.3, -3.7, -4.5, and -4.3 kcal/moL, respectively, and those of FGFC1 to KR1-KR5 were -7.4, -9.0, -6.3, -8.3, and -6.7 kcal/moL, respectively. The findings demonstrate that both EACA and FGFC1 bound to KR1-KR5 with moderately high affinity. This study could provide a theoretical basis for the clinical pharmacology of FGFC1 and establish a foundation for practical applications of FGFC1.
Keywords: FGFC1; fibrinolytic properties; molecular docking; plasminogen; pro-uPA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Fungi Fibrinolytic Compound 1 Plays a Core Role in Modulating Fibrinolysis, Altering Plasma Clot Structure, and Promoting Susceptibility to Lysis.Pharmaceutics. 2023 Sep 14;15(9):2320. doi: 10.3390/pharmaceutics15092320. Pharmaceutics. 2023. PMID: 37765289 Free PMC article.
-
A Novel Marine Pyran-Isoindolone Compound Enhances Fibrin Lysis Mediated by Single-Chain Urokinase-Type Plasminogen Activator.Mar Drugs. 2022 Jul 30;20(8):495. doi: 10.3390/md20080495. Mar Drugs. 2022. PMID: 36005498 Free PMC article.
-
Effects of a novel marine natural product: pyrano indolone alkaloid fibrinolytic compound on thrombolysis and hemorrhagic activities in vitro and in vivo.Arch Pharm Res. 2015 Aug;38(8):1530-40. doi: 10.1007/s12272-014-0518-y. Epub 2014 Dec 5. Arch Pharm Res. 2015. PMID: 25475097
-
Endothelium and disordered fibrin turnover in the injured lung: newly recognized pathways.Crit Care Med. 2002 May;30(5 Suppl):S274-80. doi: 10.1097/00003246-200205001-00017. Crit Care Med. 2002. PMID: 12004248 Review.
-
Coagulation, fibrinolysis, and fibrin deposition in acute lung injury.Crit Care Med. 2003 Apr;31(4 Suppl):S213-20. doi: 10.1097/01.CCM.0000057846.21303.AB. Crit Care Med. 2003. PMID: 12682443 Review.
Cited by
-
Fungi Fibrinolytic Compound 1 Plays a Core Role in Modulating Fibrinolysis, Altering Plasma Clot Structure, and Promoting Susceptibility to Lysis.Pharmaceutics. 2023 Sep 14;15(9):2320. doi: 10.3390/pharmaceutics15092320. Pharmaceutics. 2023. PMID: 37765289 Free PMC article.
-
Novel Deep Sea Isoindole Alkaloid FGFC1 Exhibits Its Fibrinolytic Effects by Inhibiting Thrombin-Activatable Fibrinolysis Inhibitor.Pharmaceuticals (Basel). 2024 Oct 20;17(10):1401. doi: 10.3390/ph17101401. Pharmaceuticals (Basel). 2024. PMID: 39459040 Free PMC article.
-
A Novel Marine Pyran-Isoindolone Compound Enhances Fibrin Lysis Mediated by Single-Chain Urokinase-Type Plasminogen Activator.Mar Drugs. 2022 Jul 30;20(8):495. doi: 10.3390/md20080495. Mar Drugs. 2022. PMID: 36005498 Free PMC article.
-
Recent advancement of novel marine fungi derived secondary metabolite fibrinolytic compound FGFC in biomedical applications: a review.Front Cell Infect Microbiol. 2024 Sep 18;14:1422648. doi: 10.3389/fcimb.2024.1422648. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39359937 Free PMC article. Review.
-
Progress in Isoindolone Alkaloid Derivatives from Marine Microorganism: Pharmacology, Preparation, and Mechanism.Mar Drugs. 2022 Jun 20;20(6):405. doi: 10.3390/md20060405. Mar Drugs. 2022. PMID: 35736208 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

