3D Model Characterization by 2D and 3D Imaging in t(14;18)-Positive B-NHL: Perspectives for In Vitro Drug Screens in Follicular Lymphoma
- PMID: 33804934
- PMCID: PMC8036410
- DOI: 10.3390/cancers13071490
3D Model Characterization by 2D and 3D Imaging in t(14;18)-Positive B-NHL: Perspectives for In Vitro Drug Screens in Follicular Lymphoma
Abstract
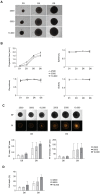
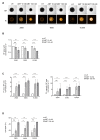
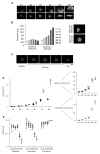
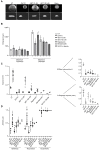
Follicular lymphoma (FL) is an indolent B cell lymphoproliferative disorder of transformed follicular center B cells, which accounts for 20-30 percent of all non-Hodgkin lymphoma (NHL) cases. Great advances have been made to identify the most relevant targets for precision therapy. However, no relevant models for in vitro studies have been developed or characterized in depth. To this purpose, we generated a 3D cell model from t(14;18)-positive B-NHL cell lines cultured in ultra-low attachment 96-well plates. Morphological features and cell growth behavior were evaluated by classical microscopy (2D imaging) and response to treatment with different drugs was evaluated by a high-content analysis system to determine the robustness of the model. We show that the ultra-low attachment (ULA) method allows the development of regular, spherical and viable ULA-multicellular aggregates of lymphoma cells (MALC). However, discrepancies in the results obtained after 2D imaging analyses on drug-treated ULA-MALC prompted us to develop 3D imaging and specific analyses. We show by using light sheet microscopy and specifically developed 3D imaging algorithms that 3D imaging and dedicated analyses are necessary to characterize morphological properties of 3D models and drug effects. This study proposes a new method, but also imaging tools and informatic solutions, developed for FL necessary for future preclinical studies.
Keywords: 2D imaging; 3D model; SPIM; drug testing; follicular lymphoma; spheroid.
Conflict of interest statement
R.M., M.N., A.G. and J.-M.L are employees of Imactiv3D.
Figures


Similar articles
-
Anti-tumor activity of obinutuzumab and rituximab in a follicular lymphoma 3D model.Blood Cancer J. 2013 Aug 9;3(8):e131. doi: 10.1038/bcj.2013.32. Blood Cancer J. 2013. PMID: 23933705 Free PMC article.
-
A novel 3D culture model recapitulates primary FL B-cell features and promotes their survival.Blood Adv. 2021 Dec 14;5(23):5372-5386. doi: 10.1182/bloodadvances.2020003949. Blood Adv. 2021. PMID: 34555842 Free PMC article.
-
T follicular helper cells: a potential therapeutic target in follicular lymphoma.Oncotarget. 2017 Nov 30;8(67):112116-112131. doi: 10.18632/oncotarget.22788. eCollection 2017 Dec 19. Oncotarget. 2017. PMID: 29340116 Free PMC article. Review.
-
Comparative Analysis of 3D Bladder Tumor Spheroids Obtained by Forced Floating and Hanging Drop Methods for Drug Screening.Front Physiol. 2017 Aug 22;8:605. doi: 10.3389/fphys.2017.00605. eCollection 2017. Front Physiol. 2017. PMID: 28878686 Free PMC article.
-
Hematopoietic cell transplantation for diffuse large B-cell and follicular lymphoma: Current controversies and advances.Hematol Oncol Stem Cell Ther. 2017 Dec;10(4):277-284. doi: 10.1016/j.hemonc.2017.05.004. Epub 2017 Jun 13. Hematol Oncol Stem Cell Ther. 2017. PMID: 28633038 Review.
Cited by
-
Patient-derived lymphoma spheroids integrating immune tumor microenvironment as preclinical follicular lymphoma models for personalized medicine.J Immunother Cancer. 2023 Oct;11(10):e007156. doi: 10.1136/jitc-2023-007156. J Immunother Cancer. 2023. PMID: 37899130 Free PMC article.
-
Three-dimensional models: a novel approach for lymphoma research.J Cancer Res Clin Oncol. 2022 Apr;148(4):753-765. doi: 10.1007/s00432-021-03897-9. Epub 2022 Jan 29. J Cancer Res Clin Oncol. 2022. PMID: 35091834 Free PMC article. Review.
-
Emerging Strategies in 3D Culture Models for Hematological Cancers.Hemasphere. 2023 Jul 27;7(8):e932. doi: 10.1097/HS9.0000000000000932. eCollection 2023 Aug. Hemasphere. 2023. PMID: 37520775 Free PMC article. Review.
-
Modeling the crosstalk between malignant B cells and their microenvironment in B-cell lymphomas: challenges and opportunities.Front Immunol. 2023 Nov 2;14:1288110. doi: 10.3389/fimmu.2023.1288110. eCollection 2023. Front Immunol. 2023. PMID: 38022603 Free PMC article. Review.
-
Comparing the value of mono- vs coculture for high-throughput compound screening in hematological malignancies.Blood Adv. 2023 Oct 10;7(19):5925-5936. doi: 10.1182/bloodadvances.2022009652. Blood Adv. 2023. PMID: 37352275 Free PMC article.
References
-
- Sutherland R.M., McCredie J.A., Inch W.R. Growth of Multicell Spheroids in Tissue Culture as a Model of Nodular Carcinomas. J. Natl. Cancer Inst. 1971;46:113–120. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

