GSK-3 and Tau: A Key Duet in Alzheimer's Disease
- PMID: 33804962
- PMCID: PMC8063930
- DOI: 10.3390/cells10040721
GSK-3 and Tau: A Key Duet in Alzheimer's Disease
Abstract
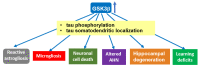
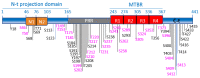
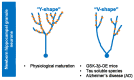
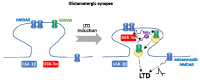
Glycogen synthase kinase-3 (GSK-3) is a ubiquitously expressed serine/threonine kinase with a plethora of substrates. As a modulator of several cellular processes, GSK-3 has a central position in cell metabolism and signaling, with important roles both in physiological and pathological conditions. GSK-3 has been associated with a number of human disorders, such as neurodegenerative diseases including Alzheimer's disease (AD). GSK-3 contributes to the hyperphosphorylation of tau protein, the main component of neurofibrillary tangles (NFTs), one of the hallmarks of AD. GSK-3 is further involved in the regulation of different neuronal processes that are dysregulated during AD pathogenesis, such as the generation of amyloid-β (Aβ) peptide or Aβ-induced cell death, axonal transport, cholinergic function, and adult neurogenesis or synaptic function. In this review, we will summarize recent data about GSK-3 involvement in these processes contributing to AD pathology, mostly focusing on the crucial interplay between GSK-3 and tau protein. We further discuss the current development of potential AD therapies targeting GSK-3 or GSK-3-phosphorylated tau.
Keywords: Alzheimer’s disease; GSK-3; neurodegeneration; tau phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration.Acta Neuropathol. 2002 Dec;104(6):583-91. doi: 10.1007/s00401-002-0587-8. Epub 2002 Jul 13. Acta Neuropathol. 2002. PMID: 12410379
-
Roles of glycogen synthase kinase 3 in Alzheimer's disease.Curr Alzheimer Res. 2012 Sep;9(7):864-79. doi: 10.2174/156720512802455386. Curr Alzheimer Res. 2012. PMID: 22272620 Review.
-
Role of glycogen synthase kinase-3 in Alzheimer's disease pathogenesis and glycogen synthase kinase-3 inhibitors.Expert Rev Neurother. 2010 May;10(5):703-10. doi: 10.1586/ern.10.40. Expert Rev Neurother. 2010. PMID: 20420491
-
GSK-3 is essential in the pathogenesis of Alzheimer's disease.J Alzheimers Dis. 2006;9(3 Suppl):309-17. doi: 10.3233/jad-2006-9s335. J Alzheimers Dis. 2006. PMID: 16914869 Review.
-
Axonal transport, tau protein, and neurodegeneration in Alzheimer's disease.Neuromolecular Med. 2002;2(2):151-65. doi: 10.1385/NMM:2:2:151. Neuromolecular Med. 2002. PMID: 12428809 Review.
Cited by
-
Exploring the protective effect and molecular mechanism of betulin in Alzheimer's disease based on network pharmacology, molecular docking and experimental validation.Mol Med Rep. 2024 Dec;30(6):232. doi: 10.3892/mmr.2024.13356. Epub 2024 Oct 11. Mol Med Rep. 2024. PMID: 39392030 Free PMC article.
-
Glycogen synthase kinase 3 (GSK3) inhibition: a potential therapeutic strategy for Alzheimer's disease.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar;398(3):2319-2342. doi: 10.1007/s00210-024-03500-1. Epub 2024 Oct 21. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39432068 Review.
-
Target Validation Studies of PS48, a PDK-1 Allosteric Agonist, for the Treatment of Alzheimer's Disease Phenotype in APP/PS1 Transgenic Mice.Int J Mol Sci. 2025 Apr 8;26(8):3473. doi: 10.3390/ijms26083473. Int J Mol Sci. 2025. PMID: 40331945 Free PMC article.
-
Neuroprotective potential of hispidulin and diosmin: a review of molecular mechanisms.Metab Brain Dis. 2025 Apr 21;40(5):188. doi: 10.1007/s11011-025-01615-9. Metab Brain Dis. 2025. PMID: 40257619 Review.
-
Effectiveness of epigallocatechin gallate nanoparticles on the in-vivo treatment of Alzheimer's disease in a rat/mouse model: a systematic review.Daru. 2024 Jun;32(1):319-337. doi: 10.1007/s40199-023-00494-8. Epub 2023 Dec 11. Daru. 2024. PMID: 38079104 Free PMC article.
References
-
- Rylatt D.B., Aitken A., Bilham T., Condon G.D., Embi N., Cohen P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur. J. Biochem. 1980;107:529–537. doi: 10.1111/j.1432-1033.1980.tb06060.x. - DOI - PubMed
-
- Hanger D.P., Hughes K., Woodgett J.R., Brion J.P., Anderton B.H. Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: Generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci. Lett. 1992;147:58–62. doi: 10.1016/0304-3940(92)90774-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

