CXCL12-Abundant Reticular (CAR) Cells Direct Megakaryocyte Protrusions across the Bone Marrow Sinusoid Wall
- PMID: 33804965
- PMCID: PMC8063926
- DOI: 10.3390/cells10040722
CXCL12-Abundant Reticular (CAR) Cells Direct Megakaryocyte Protrusions across the Bone Marrow Sinusoid Wall
Abstract
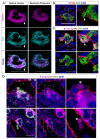
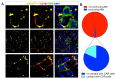
Megakaryocytes (MKs) release platelets into the lumen of bone marrow (BM) sinusoids while remaining to reside within the BM. The morphogenetic events of this complex process are still not fully understood. We combined confocal laser scanning microscopy with transmission and serial block-face scanning electron microscopy followed by 3D-reconstruction on mouse BM tissue sections. These analyses revealed that MKs in close vicinity to BM sinusoid (BMS) wall first induce the lateral retraction of CXCL12-abundant reticular (CAR) cells (CAR), followed by basal lamina (BL) degradation enabling direct MK-sinusoidal endothelial cells (SECs) interaction. Subsequently, an endothelial engulfment starts that contains a large MK protrusion. Then, MK protrusions penetrate the SEC, transmigrate into the BMS lumen and form proplatelets that are in direct contact to the SEC surface. Furthermore, such processes are induced on several sites, as observed by 3D reconstructions. Our data demonstrate that MKs in interaction with CAR-cells actively induce BMS wall alterations, including CAR-cell retraction, BL degradation, and SEC engulfment containing a large MK protrusion. This results in SEC penetration enabling the migration of MK protrusion into the BMS lumen where proplatelets that are adherent to the luminal SEC surface are formed and contribute to platelet release into the blood circulation.
Keywords: CXCL12-abundant reticular (CAR)-cells; megakaryocytes; microvasculature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Nishimura S., Nagasaki M., Kunishima S., Sawaguchi A., Sakata A., Sakaguchi H., Ohmori T., Manabe I., Italiano J.E., Ryu T., et al. IL-1 alpha induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J. Cell Biol. 2015;209:453–466. doi: 10.1083/jcb.201410052. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

